In regards to CSI/LSI within the following:
 www.troublefreepool.com
www.troublefreepool.com
it states this:
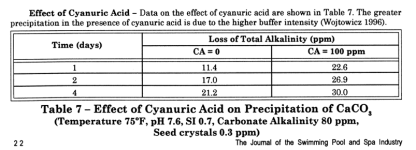
“The results can be summarized as follows: […]
“The … equation is: SI = pH + Log[Hard] + Log[Alk] + TC + C”
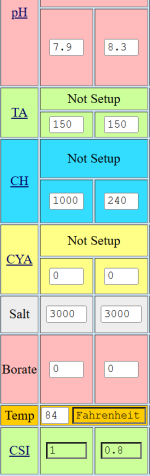
Am I reading something incorrectly?
CSI and LSI - Further Reading
it states this:
“The results can be summarized as follows: […]
- at a given initial alkalinity and SI, the extent of precipitation decreases with increasing pH”
“The … equation is: SI = pH + Log[Hard] + Log[Alk] + TC + C”
Am I reading something incorrectly?