Quote taken from the thread Chlorine after cya bond is released
If I am understanding correctly, the chlorine atom bonded to the CYA molecule is neither hypochlorous acid nor hypochlorite ion.
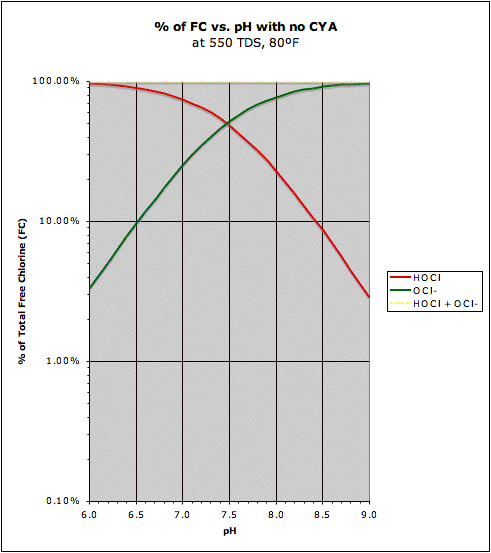
James gives an excellent formula and chart for the ratio of HCLO to CLO- given pH when CYA is not present.
My question is: Is there any way to know how that pH-ratio relationship changes given, say, a set amount of CYA?
Thanks!
With no cyanuric acid in the water, the pH does determine the percentage of hypochlorite vs. hypochlorous acid.
The pKa of hypochlorous acid is about 7.53.
1÷(1+10^(7.53 – pH)) = hypochlorite percentage.
For example:
pH..........hypochlorite percentage
8.53..........0.90909
8.00.........0.747
7.53.........0.50
7.30.........0.37
Hypochlorous acid and hypochlorite both oxidize and sanitize, but hypochlorous acid is better at both.
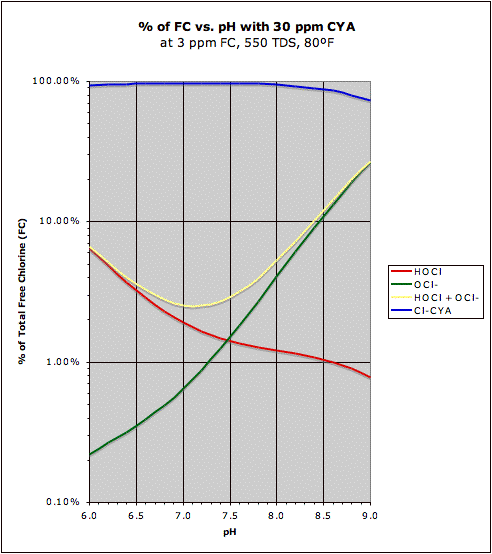
With cyanuric acid in the water, the equation changes because most of the chlorine is bound to cyanuric acid, but the pH does still effect the ratio. The effect is not as great, but it still matters somewhat.
If I am understanding correctly, the chlorine atom bonded to the CYA molecule is neither hypochlorous acid nor hypochlorite ion.
James gives an excellent formula and chart for the ratio of HCLO to CLO- given pH when CYA is not present.
My question is: Is there any way to know how that pH-ratio relationship changes given, say, a set amount of CYA?
Thanks!