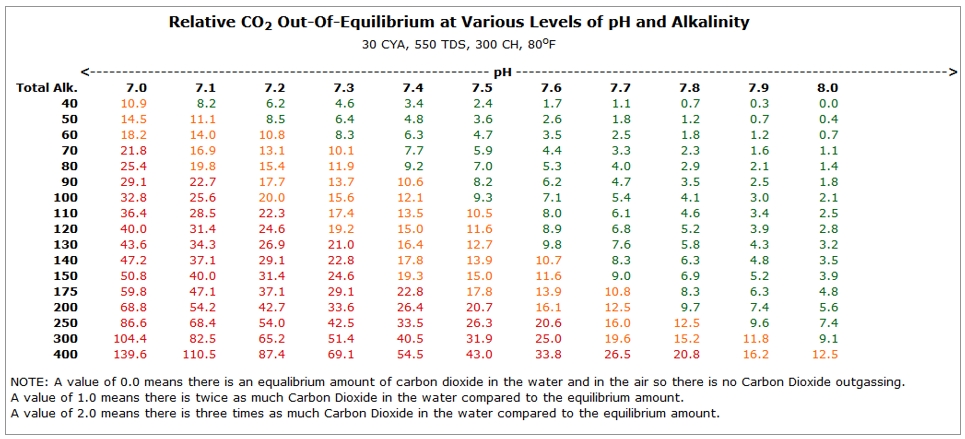
High TA creates a lot of carbon dioxide, which causes the pH to rise.
So, it is counterintuitive that a lower TA results in a more stable pH.
It is a myth that low TA causes the pH to fluctuate.
pH changes due to added base or acid.
TA really only changes how much pH change happens when you add an acid or base.
The TA can be very close to zero without the pH going wild like some people suggest.
With low TA, you just have to be more careful about what is added, especially added acid.
Ideally, you should almost never need to add acid.
So, it is counterintuitive that a lower TA results in a more stable pH.
It is a myth that low TA causes the pH to fluctuate.
pH changes due to added base or acid.
TA really only changes how much pH change happens when you add an acid or base.
The TA can be very close to zero without the pH going wild like some people suggest.
With low TA, you just have to be more careful about what is added, especially added acid.
Ideally, you should almost never need to add acid.