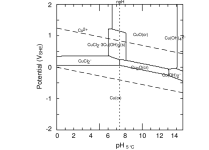
This is strictly a theoretical question, given that in most scenarios to achieve a proper LSI you're generally going to have a TA and pH that will be fine for your heater. Acknowledging that, does a copper heat exchanger care more about LSI or pH? Seems that LSI is mostly geared toward plaster longevity.
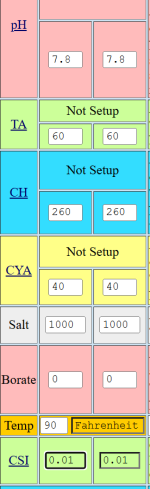
As an illustration, if my CA was very low, say 50, water temp of 90, TA of 100, CYA of 10, and Salt at 3400, pH is 7, the LSI is -1.38. I would guess that is very hard on the plaster, as the plaster wants to give calcium back to the water, but will it also be hard on the heat exchanger? The heat exchanger doesn't want to leach calcium back into the water. It doesn't have any.
Or is "etching" (negative LSI) water, etching to all materials and surfaces regardless? Or is there a strict pH band that should be maintained for your copper heater regardless, even though the LSI could theoretically still be balanced.
As an illustration, if my CA was very low, say 50, water temp of 90, TA of 100, CYA of 10, and Salt at 3400, pH is 7, the LSI is -1.38. I would guess that is very hard on the plaster, as the plaster wants to give calcium back to the water, but will it also be hard on the heat exchanger? The heat exchanger doesn't want to leach calcium back into the water. It doesn't have any.
Or is "etching" (negative LSI) water, etching to all materials and surfaces regardless? Or is there a strict pH band that should be maintained for your copper heater regardless, even though the LSI could theoretically still be balanced.