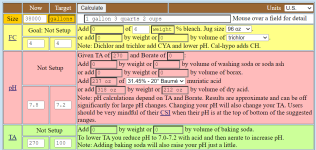
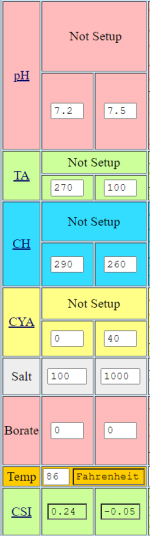
Due to illness, the belated startup of my pool happened last week where for the saturation index (at the current 69 degrees Fahrenheit & 7.6 pH), I need roughly around 3 full HASA cases of 31.45% muriatic acid (to drop alkalinity from 270 ppm to 100 ppm) and just under about 50 pounds of calcium chloride (to raise calcium hardness from 290 ppm to 400 ppm), where my only question in this thread is how long would you take to add that amount of acid incrementally?
Given it takes about a month (or so) to fill the pool shell at about an average of a thousand gallons a day (which is all the well pump & booster pump can handle), there's no chance in using any of the filtering or self-cleaning circulation system, but I'm doing just fine using home-made self-engineered ad hoc buckets.
Using buckets for dissolving and for dilution while I'm slowly filling the pool, even without the pool equipment running, I'm easily slowly incrementally dosing to the requisite 7.5% of the CYA HASA chlorine sanitation (at ~3.25 ppm) & 30 ppm endgoal CYA protection (there is infinite sunlight and no cover) & 400 Calcium hardness endgoal needs by pouring a 25% dilution of chlorine and dissolving the almost-impossible-to-dissolve CYA granules and exothermic CaCl flakes into buckets - where my main question about timing is about the dozen gallons of HCl mostly.
How long would you take to slowly incrementally add a dozen gallons of hydrochloric acid to a pool by hand?
Would you take a month? A week? A few days?
Given it takes about a month (or so) to fill the pool shell at about an average of a thousand gallons a day (which is all the well pump & booster pump can handle), there's no chance in using any of the filtering or self-cleaning circulation system, but I'm doing just fine using home-made self-engineered ad hoc buckets.
Using buckets for dissolving and for dilution while I'm slowly filling the pool, even without the pool equipment running, I'm easily slowly incrementally dosing to the requisite 7.5% of the CYA HASA chlorine sanitation (at ~3.25 ppm) & 30 ppm endgoal CYA protection (there is infinite sunlight and no cover) & 400 Calcium hardness endgoal needs by pouring a 25% dilution of chlorine and dissolving the almost-impossible-to-dissolve CYA granules and exothermic CaCl flakes into buckets - where my main question about timing is about the dozen gallons of HCl mostly.
How long would you take to slowly incrementally add a dozen gallons of hydrochloric acid to a pool by hand?
Would you take a month? A week? A few days?