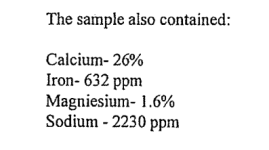
For the past year, sediment has built up in the heat exchanger, it needs to be cleaned out before the heater will restart. The company we work with had the solid "blue chips" analyzed and it was determined that the makeup was: SULFATE 1070 Mg/kg, Nickel 987 ppm, Copper 8159 ppm, and Chloride 1124 ppm. The pool water was also analyzed and the contents were: SULFATE 16 mg/L, Nickel <0.01 mg/L, Copper <0.01 mg/L, and Chloride 847 mg/L.
The source water has been changed from well to public, Pool Metal Magnet has been added regularly for years due to the metals in the well water. The heat exchanger was changed from a copper/nickel blend to a pure copper part and the result has produced very little if any change. Recently when the heat exchanger was cleaned the substance was a clay-like substance versus the chip substance that was previously found.
The heater was replaced about a year ago and is a Raypak. The heat exchanger on the old heater, also a Raypak, was cut open and the same substance was found in it. The old heater did not have the fail-safe that would not allow the heater to be turned back on unless the exchanger had been cleaned out. This is an indoor pool and it is open year-round. The clogs happen anywhere from one week to three weeks after cleaning.
Ideas, thoughts, solutions?
I appreciate your help,
Emily
The source water has been changed from well to public, Pool Metal Magnet has been added regularly for years due to the metals in the well water. The heat exchanger was changed from a copper/nickel blend to a pure copper part and the result has produced very little if any change. Recently when the heat exchanger was cleaned the substance was a clay-like substance versus the chip substance that was previously found.
The heater was replaced about a year ago and is a Raypak. The heat exchanger on the old heater, also a Raypak, was cut open and the same substance was found in it. The old heater did not have the fail-safe that would not allow the heater to be turned back on unless the exchanger had been cleaned out. This is an indoor pool and it is open year-round. The clogs happen anywhere from one week to three weeks after cleaning.
Ideas, thoughts, solutions?
I appreciate your help,
Emily