- Sep 23, 2015
- 2,012
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V25
My apologies to any all who have followed my postings with resulting confusion and/or frustration with my ignorance, sometimes amplified by some of my muddled explanations. I'm sure there are many members with vastly more knowledge and ability than I in chemistry and mathematics for whom my ignorance could lead to frustration.
I should have clearly stated where I was coming from and what I was hoping to learn, and the approach I had taken to find some answers for myself.
What I wanted to find out:
How can I figure out, under whatever combinations of CYA, FC and pH, what percentage (fraction) of each Mole of chlorine in the pool is in the forms of HOCl and OCl-? How do those results change as you change those conditions? Allowing me then to do some comparative analysis. For me, this is not a purely scholarly pursuit. The answers to my questions will help my decision making in operating our 2 semi-public pools, hopefully better, more efficiently and economically. And within County Health Code operating boundaries.
So - Research the problem. I quickly found some guideline estimates like "98% of the chlorine is bound to CYA", approximately 50% unbound chlorine at pH 7.5 is in the form of HOCl. I read, downloaded a lot of information I located that ChemGeek had posted on TFP, as well as from other sites or referenced documents online, that might be applicable and analyzed that info. Read and downloaded a lot of basic research papers, including the O'Brien papers, that presented elements that I felt were relevant to swimming pools, and try as best I could to understand those. Most of the time having to Google for terminology definitions, chemical reactions, etc.
Anyway, after many untutored attempts at calculating (in other words, getting myself wrapped around the axle), I happened upon the EPA (Wahman) calculator app. Aha! Thought I.
However, the app generates all its values as the negative Log of the molar concentrations (mol/l) at the given conditions. OK. I'll presume they're accurate, as they're based on the prior work of O'Brien, et al. Now, how to separate out just the fractional molar relationships from the concentrations?
Ultimately, I figured out a way to derive the Molar Fraction from the negative Log of the Molar Concentration, at least for total free chlorine, HOCl and OCl-. For all the rest, I'll have to learn how to use the negative log molar concentration values directly. That is, if I ever really need to know something like what fraction of each initial mole of TriChlor (Cl3CY) in solution is in the form of H2ClCy under various conditions.
Being able to calculate the Molar Fractions at different FC, CYA and pH conditions, allowed me to solve, at least for myself, how and why Mark's test results came out as they did, and the implications of that for our pool operations.
Thanks for your patience.
Hi Tom,
Thank you for sharing, there are not too many people that would have the time or patience to dig as deep as you have, most just want to jump in and relax. I kept thinking ”but why”, and then there it is. But I’m thinking maybe it would be easier to approach this in a less precise manner using historical data.
Its like your trying to count and separate sand grains by size in a sand storm in order to ensure there is more of one size than the other according to a theoretical ratio that is constantly affected by random deviation. A wise man once said, actually it was Jim from above, that it is more like a game of grenades then a game of darts. Pools never reach equilibrium, they are always in state constant change and our testing lacks the precision to be super precise. And for most of us that level of precision is just not needed anyway. Any test is just a snap shot in time at that precise moment, it doesn’t tell us an enormous amount about where the pool was a week ago and where it will be in a weeks time.
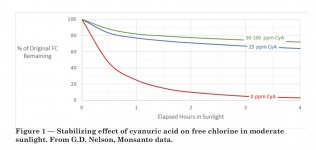
There is also a major lack of literature and research. I looked though an old first year chemistry text book and in over 1200 pages the term cyanuric acid doesn’t even appear once. And I could probably count on one hand the number of published articles relating to cyanuric acid and the relationship to FC. Its sad to think that we know more about so many obscure things that are mostly meaningless to the general public then we know about the simple interaction of a handful of chemical species in the average backyard pool that is part of one of the largest industries worldwide.
Having said that it does appear that the pool industry (domestic and commercial) lacks the people who are willing to think outside of the box, to test the boundaries and to achieve more, so knock yourself out, dig as deep as your willing.