During the summer of 2021 we switched from using 3-inch Trichlor tablets in Pentair feeders to peristaltic pumps using 10% - 12.5% Sodium Hypochlorite (Bleach) hoping to reduce operating costs and to save water (periodically having to dump a lot of water due to constantly rising CYA). At peak summer we had typically used about 3.88 PPM chlorine per day in each of our 2 approximately 32,500 semi-public pools here in Tucson, AZ, averaging 2.33 8-oz Trichlor tablets/day/pool. After the conversion to Bleach we noted we were having to use substantially more Bleach than we had estimated, based on the 12.8 fl oz/PPM/10,000 gallons (10% Cl2 equivalent) vs 1.48 oz Trichlor/PPM/10,000 gallons guidance.
As a result, I started trying to figure out what was causing the higher-than-expected Bleach use. It wasn’t “weak” Bleach solution. I started testing the chlorine content and found it was as advertised. During my “research” I found a posting on the TFP forum by member mas985, dated 7/1/21, titled “CYA vs UV Chlorine Loss Test – Updated”.
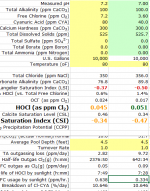
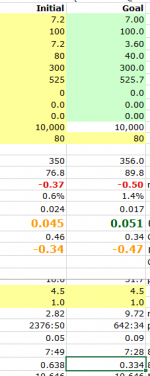
A Recap of Test Conditions presented by mas985 of the single sample source pool water for the initial condition and subsequent dilutions, and the results:
Initial Sample, FC 7.2, CYA 80, FC/CYA Ratio .09, pH 7.2.
Resulting 2-hour FC PPM Loss .4 PPM
1st Dilution 1:1, FC 3.6, CYA 40, FC/CYA Ratio .09, pH 7.02 (adjusted from dilution with pH 6.9 distilled water)
Resulting 2-hour FC PPM Loss 1 PPM
2nd Dilution 1:3, FC 1.8, CYA 20, FC/CYA Ratio .09, pH 6.98 (adjusted from dilution with pH 6.9 distilled water)
Resulting 2-hour FC PPM Loss 1.4 PPM
Initial, 1st and 2nd dilutions were in open-top clear containers placed in mid-day summer sun for 2 hours.
The first thing I noticed was the critical role of CYA in shielding the chlorine from solar UV burn-off.
At one-half the CYA concentration (CYA 40), the FC loss was about 2 times that of the Initial of 80 PPM CYA sample. At one-quarter CYA concentration (CYA 20) the FC loss was about 4 times that of the Initial condition of 80 PPM CYA.
But, note that the FC/CYA ratio is exactly the same in each prepared sample.
Of considerable help to me in understanding the CYA – Chlorine relationship was a web-based application I had found: “Free Chlorine and Cyanuric Acid System Simulator”, created by David G Wahman, U.S. EPA. The tool is based on the equilibrium model presented by O'Brien (1972) and O'Brien et al (1974). Accessible at: https://usepaord.shinyapps.io/cyanuric/ . The application generates graphic representation of resulting values and can generate and download to your computer a CSV file of computed results. Note: All chemical relationships are at 25 °C.
The Application's results are given as negative log molar concentrations. So, you need to figure out the math and conversions.
EPA Application Definitions and Terminology Equivalents:
Total Chlorine: CYA-Bound + Unbound Chlorine = DPD tested Free Chlorine (FC)
Total Free Chlorine: Chlorine Unbound from CYA, HOCl + OCl-. Can’t be directly tested, has to be computed, and is the concentration of the chlorine exposed to Solar UV. Equivalent to Free Available Chlorine (FAC).
Total Cyanuric = Stated CYA concentration
Resultant data points for the 3 test conditions:
Initial 1st Dilution 2nd Dilution
FAC Molar Concentration 0.009295707 0.019057203 0.037339955
FAC PPM 0.066929089 0.068605932 0.067211919
Reported 2-hr FC PPM Loss .4 1.0 1.4
Computed FC PPM Loss .4 .820 1.607
I attribute the bulk of the variance between Reported and Computed FC Loss to the +/- .2 PPM accuracy range of the FAS-DPD test, along with some small variance due to the inability to represent lesser than a .1 pH precision in the computational simulator.
Observations:
At the constant FC/CYA Ratio of .09, the starting FAC PPM remains essentially flat across the Initial, 1st and 2nd dilutions tested.
The FC PPM Loss rate is directly proportional to the FAC Molar Concentration, not the FAC PPM.
Therefore, I believe a Rate Constant loss is present: R * FAC Molar Concentration.
I calculated the 2-hr FC PPM loss Rate in this test as 43.0306 * FAC Molar Concentration across all three test solutions. The Rate Constant expression is dependent on the amount of clear-sky solar insolation, which varies throughout the year and by geographic latitude.
The FC/CYA Ratio is highly correlated to maintaining adequate HOCl PPM for disinfection:
Initial 1st Dilution 2nd Dilution
HOCl PPM (computed) 0.045933451 0.053248808 0.052166838
Note the increased HOCl PPM in 1st and 2nd Dilutions due to the reduced pH of those solutions having shifted the HOCl/OCl- ratio a bit more toward HOCl.
The mas985 test was a “static” test over a two-hour period – declining balance at half-life intervals such as the approximate 12-min interval of OCl- over a 2-hr period, I assume that a “dynamic” constant-feed of chlorine will result in a higher chlorine loss at any given CYA level, particularly when adding unstabilized chlorine as with 10% – 12% pool bleach or with a SWCG, versus using Trichlor, which will continuously and progressively change the FC/CYA ratio.
Caveat:
My calculated FC PPM Loss Rate Constant of 43.0306 * FAC Molar Concentration for the mas985 test samples group is Not Applicable to a real-life swimming pool situation, in which the ratio of exposed pool surface area in square feet / the total cubic feet of water will have significant effect on the calculated loss rate of the full FAC content in the pool, due to UV shielding at depth from the limited UV penetration of water.
However, for me, this was an eye-opener into the mechanism of solar UV chlorine loss and the validity of the previously established FC/CYA ratios.
As a result, I started trying to figure out what was causing the higher-than-expected Bleach use. It wasn’t “weak” Bleach solution. I started testing the chlorine content and found it was as advertised. During my “research” I found a posting on the TFP forum by member mas985, dated 7/1/21, titled “CYA vs UV Chlorine Loss Test – Updated”.
A Recap of Test Conditions presented by mas985 of the single sample source pool water for the initial condition and subsequent dilutions, and the results:
Initial Sample, FC 7.2, CYA 80, FC/CYA Ratio .09, pH 7.2.
Resulting 2-hour FC PPM Loss .4 PPM
1st Dilution 1:1, FC 3.6, CYA 40, FC/CYA Ratio .09, pH 7.02 (adjusted from dilution with pH 6.9 distilled water)
Resulting 2-hour FC PPM Loss 1 PPM
2nd Dilution 1:3, FC 1.8, CYA 20, FC/CYA Ratio .09, pH 6.98 (adjusted from dilution with pH 6.9 distilled water)
Resulting 2-hour FC PPM Loss 1.4 PPM
Initial, 1st and 2nd dilutions were in open-top clear containers placed in mid-day summer sun for 2 hours.
The first thing I noticed was the critical role of CYA in shielding the chlorine from solar UV burn-off.
At one-half the CYA concentration (CYA 40), the FC loss was about 2 times that of the Initial of 80 PPM CYA sample. At one-quarter CYA concentration (CYA 20) the FC loss was about 4 times that of the Initial condition of 80 PPM CYA.
But, note that the FC/CYA ratio is exactly the same in each prepared sample.
Of considerable help to me in understanding the CYA – Chlorine relationship was a web-based application I had found: “Free Chlorine and Cyanuric Acid System Simulator”, created by David G Wahman, U.S. EPA. The tool is based on the equilibrium model presented by O'Brien (1972) and O'Brien et al (1974). Accessible at: https://usepaord.shinyapps.io/cyanuric/ . The application generates graphic representation of resulting values and can generate and download to your computer a CSV file of computed results. Note: All chemical relationships are at 25 °C.
The Application's results are given as negative log molar concentrations. So, you need to figure out the math and conversions.
EPA Application Definitions and Terminology Equivalents:
Total Chlorine: CYA-Bound + Unbound Chlorine = DPD tested Free Chlorine (FC)
Total Free Chlorine: Chlorine Unbound from CYA, HOCl + OCl-. Can’t be directly tested, has to be computed, and is the concentration of the chlorine exposed to Solar UV. Equivalent to Free Available Chlorine (FAC).
Total Cyanuric = Stated CYA concentration
Resultant data points for the 3 test conditions:
Initial 1st Dilution 2nd Dilution
FAC Molar Concentration 0.009295707 0.019057203 0.037339955
FAC PPM 0.066929089 0.068605932 0.067211919
Reported 2-hr FC PPM Loss .4 1.0 1.4
Computed FC PPM Loss .4 .820 1.607
I attribute the bulk of the variance between Reported and Computed FC Loss to the +/- .2 PPM accuracy range of the FAS-DPD test, along with some small variance due to the inability to represent lesser than a .1 pH precision in the computational simulator.
Observations:
At the constant FC/CYA Ratio of .09, the starting FAC PPM remains essentially flat across the Initial, 1st and 2nd dilutions tested.
The FC PPM Loss rate is directly proportional to the FAC Molar Concentration, not the FAC PPM.
Therefore, I believe a Rate Constant loss is present: R * FAC Molar Concentration.
I calculated the 2-hr FC PPM loss Rate in this test as 43.0306 * FAC Molar Concentration across all three test solutions. The Rate Constant expression is dependent on the amount of clear-sky solar insolation, which varies throughout the year and by geographic latitude.
The FC/CYA Ratio is highly correlated to maintaining adequate HOCl PPM for disinfection:
Initial 1st Dilution 2nd Dilution
HOCl PPM (computed) 0.045933451 0.053248808 0.052166838
Note the increased HOCl PPM in 1st and 2nd Dilutions due to the reduced pH of those solutions having shifted the HOCl/OCl- ratio a bit more toward HOCl.
The mas985 test was a “static” test over a two-hour period – declining balance at half-life intervals such as the approximate 12-min interval of OCl- over a 2-hr period, I assume that a “dynamic” constant-feed of chlorine will result in a higher chlorine loss at any given CYA level, particularly when adding unstabilized chlorine as with 10% – 12% pool bleach or with a SWCG, versus using Trichlor, which will continuously and progressively change the FC/CYA ratio.
Caveat:
My calculated FC PPM Loss Rate Constant of 43.0306 * FAC Molar Concentration for the mas985 test samples group is Not Applicable to a real-life swimming pool situation, in which the ratio of exposed pool surface area in square feet / the total cubic feet of water will have significant effect on the calculated loss rate of the full FAC content in the pool, due to UV shielding at depth from the limited UV penetration of water.
However, for me, this was an eye-opener into the mechanism of solar UV chlorine loss and the validity of the previously established FC/CYA ratios.