I tried aeration for a week and got nothing.Soda ash is a big hammer when it comes to adding alkalinity (and raising pH). High TA, higher CH, etc can cause clouding.
Best to not use Soda ash. Better is to use baking soda to raise TA (if necessary) and aeration to raise pH.
Had my jets facing up, if I had to add water I use my old flosser head for cartridge cleaning and made a ton or air bubbles.
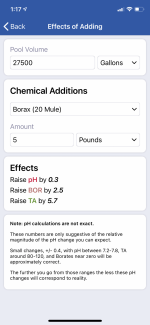
no luck, that’s why I went to soda ash. App said to use 5 pounds. I put in about 4,