Accuracy eh? How accurate or precise do we need to be with pool testing? This fills my waking days this question does so I'll chime in a little here. I live in two camps with this one, because obviously when I'm making something like a reagent it's got to be super accurate for so many reasons. When I'm actually using the kit though, I'm a lot more relaxed. I hope I'm not opening too big a can of worms with this one

????
Massive amounts of caveats go here ?? the biggest one being this is all very much just my opinion
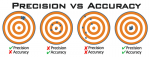
I feel like accuracy vs precision is an important distinction to make here. Accuracy being how close our result is to reality, and precision being the variation you get when repeating a test. Both would be ideal, but if I had to sacrifice one it'd be precision. This is why I think test kits are built with ease in mind, so you do the tests more frequently and get the benefit of averaging and accuracy via statistics. A bunch of loosely grouped shots at goal are more likely to hit than a bunch of tightly clustered shots not on goal.
We assume then, based on how chemistry works, that a drop test done enough times is going to approach accuracy. Between averaging data points and the fact a set number of
blah blah bits of chemistry talk bond to a set number of
super tiny blehs blehs in each microlitre and cause a colour change, we get results that approach the actual reality of the chemicals. Precision (variation) is harder and more costly to achieve as you get down to the pointy end, and things outside kit makers control (like temperature etc) become more important. Accuracy is cheaper and IMO more effective.
If you're testing with high precision (consistently getting a similar result) but that result is wrong, you'll always be out. You'll see data movement, and you'll know when your TA rises and your CYA drops and vaguely by how much, but you'll never know that it's actually x-20% for example.
Conversely if you test a lot, even if each one is done quickly or you're more of a "near enoughs good enough" person, then the extra data points you've got will smooth the data out. Assuming you're still following the instructions and staying vaguely within the lines

Any imprecision caused by these quicker test methods will be reduced by the accuracy/averaging of more data points. A good test kit should be designed to encourage repetition and therefore accuracy rather than precision, and also reduce any variance/imprecision as much as possible. Again, that's my opinion but if Taylor wants to call me and argue for some reason, then I'm sure they can

The dream of course, is a high precision, high accuracy test. Chemical drop tests take care of a lot of the precision, because x number of ions swirling around in the jigamarator will always bond with y number of thingos on the z axis and make the pretty colours happen (sorry if I lost anyone with that technical jargon). So why I love this thread so much is that it's trying to find the best way of ensuring the right amount of chemicals meet in the magic colour changing tubes. Kit designers then have to balance that with repeatibility and cost, and in a way that encourages repetition and accuracy, which is a whole other set of challenges.