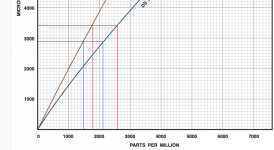
If the TDS is mostly as estimated, the salinity reading from a conductivity meter is estimated to be about 1.50 to 1.78 grams per liter (1,500 ppm to 1,780 ppm) in the salinity setting or about 2,100 ppm to 2,600 ppm in the total dissolved solids setting.
If the conductivity meter reads higher than these numbers, then there are likely to be unaccounted for dissolved solids.
If the readings are in this range, then you have likely accounted for most of the dissolved solids.
In addition to the fill water, you can do the same process for the pool water to see if you can find a suitable balanced equation.
You would include all known additions like chlorine and acid since you know how much of these you have added.
For example, you know how much acid is used, so you can calculate the number of moles of sulfate added by the acid that was added etc.

If the conductivity meter reads higher than these numbers, then there are likely to be unaccounted for dissolved solids.
If the readings are in this range, then you have likely accounted for most of the dissolved solids.
In addition to the fill water, you can do the same process for the pool water to see if you can find a suitable balanced equation.
You would include all known additions like chlorine and acid since you know how much of these you have added.
For example, you know how much acid is used, so you can calculate the number of moles of sulfate added by the acid that was added etc.


Last edited:









