For those of us in the dark, what happens if bleach comes into contact with MA
A cloud of chlorine gas.
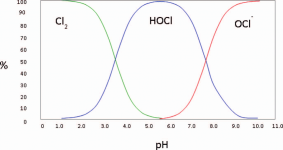
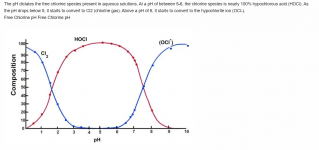
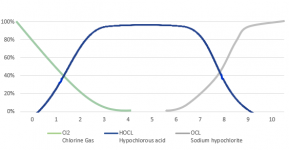
The lower the pH, the more chlorine gas is produced.
At a pH of 5.5, chlorine gas begins to be produced, at a pH of 3.5, the chlorine is about 50% chlorine gas.
At a pH of 1.0, all chlorine is converted into chlorine gas.
Different charts show different graphs.
The pH probably needs to get to about 3 to generate a lot of chlorine gas.
Liquid chlorine has a high pH and MA has a low pH, so you have to know the pH of a mixture to know how much gas is produced.
IN any case, never mix them.
Bleach and ammonia forms nitrogen trichloride NCl
3, which is a highly toxic dangerous and explosive chlorinated gas, which is at least as dangerous or more dangerous than chlorine gas.






