is it worth considering an alternative oxidiser for this initial clean up?
Without knowing what the contaminant is, it is hard to tell what would be the most effective way to get it removed.
I think that UV would be helpful.
The other alternatives are hydrogen peroxide, MPS and ozone.
I do not know if any of these would be useful or just a waste of money.
Without knowing the exact chemicals that we are dealing with, we are just guessing at what might work.
If the chlorine is getting used up, then it is probably oxidizing the chemicals, but we do not know how much total chemical or chemicals are in the water.
A bucket test would help.
Chlorine dioxide might work, but this is not typically used and you would probably not find anyone locally that would be able to do this.
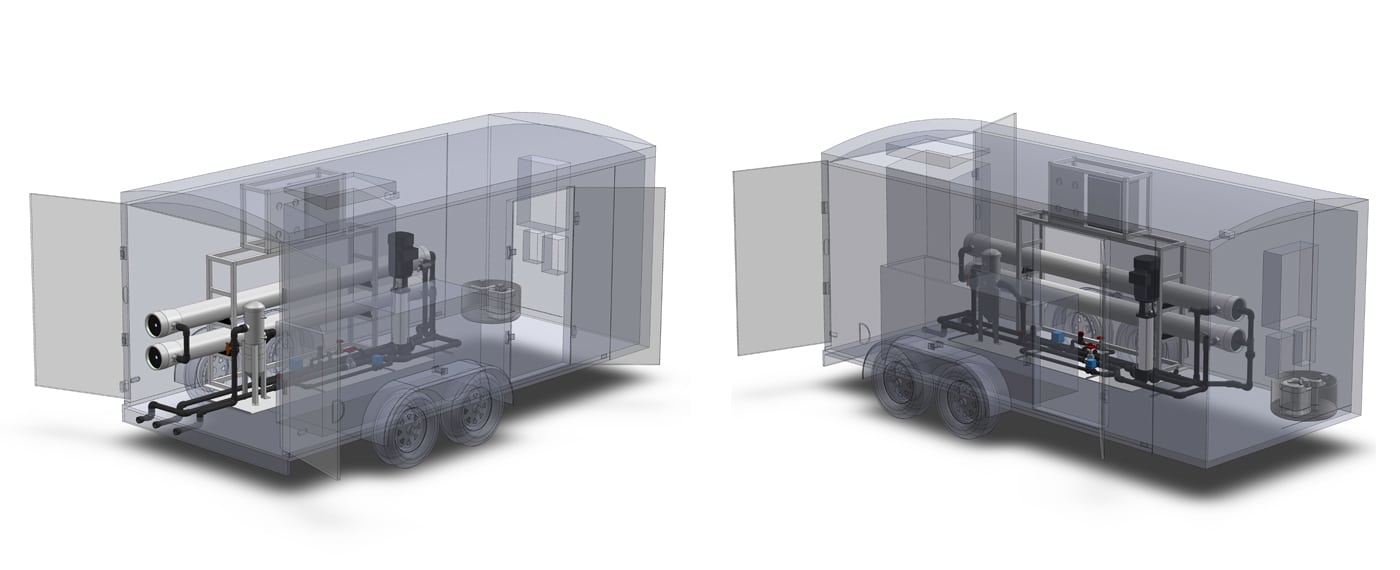
Maybe some type of commercial grade filtration with granular activated carbon or even RO treatment would work.
Some places have a RO commercial treatment that will show up and treat the entire pool.
However the services are very limited to specific areas.
Pool Filtration Reverse Osmosis Systems by Aqua Clear Water Treatment Specialists an oem manufacture of reverse osmosis and water treatment equipment.

www.watertreatmentspecialists.com