Pool Chemistry is fairly simple for chemists. We provide the chemical equations related to adding and using chlorine in a pool to educate our members and provide transparency that no pool store or maintenance can or will supply. Adding bleach is a basic process (it Raises pH); using up bleach (chlorine) is an acidic process (it lowers pH), so the net result is almost neutral. Some of this content was originally posted in the thread "Pool Water Chemistry" by user "Chem Geek". The thread is linked at the bottom of this page. Chlorine usage is the process of using chlorine to sanitize the water.
Chlorine Pool Chemistry Equations
What your test kit measures
Free Chlorine (FC) measures all the forms of chlorine in the pool, HOCl (hypochlorous acid) + OCl- (hypochlorite ion) and all the chlorine that is bound with cyanuric acid. Combined Chlorine (cc) measures the amount of free chlorine that has bound itself to a contaminant or organic material , such as ammonia or other contaminants, and is no longer available to disinfect.
Notations: (s) = solid (aq) = aqueous (g) = gas (l) = liquid
Adding Liquid Chlorine
Na++ OCl- + H2O → Na+ + HOCl + OH-
HOCl + OH- ⇌ OCl- + H2O
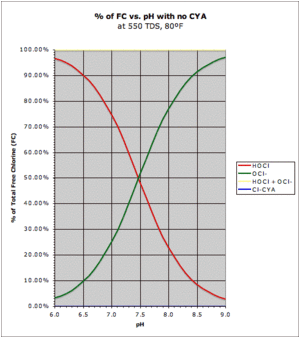
Sodium Hypochlorite (liquid chlorine or bleach) comes as a solution of sodium ions, hypochlorite ions and water. The hypochlorite ions react with water to produce sodium ions, hypochlorous acid and hydroxyl ions. Hypochlorous acid and hypochlorite ion are in an equilibrium reaction with the hypochlorite ion and water (second equation). This reaction and the concentrations on both sides are dependent on the pH where the pKa value is given as 7.54 which is the point where the concentrations of either are equal.
This graph shows the relationship in the percentages of HOCl and OH- which, because they are in equilibrium, will always total 100%.
The hydroxyl ion makes this a basic reaction that raises the pH, but because hypochlorous acid is a weak acid the overall reaction raises the pH by less than a strong base would. Note that there is a small amount of extra base in the form of Sodium Hydroxide (lye or caustic soda) that comes with Sodium Hypochlorite and is there to help preserve it, but this amount is rather small. Because of the Sodium ion addition, when you add liquid chlorine to the pool, you are adding salt.
Using Up Of Chlorine
Breakdown of Chlorine by Sunlight (UV)
2HOCl → O2(g) + 2H+ + 2Cl-
2OCl- → O2(g) + 2Cl-
Chlorine breaks down in the presence of ultraviolet radiation, such as in sunlight, and forms oxygen gas and chloride ion (and hydrogen ion, if starting with HOCl hypochlorite). Because a hydrogen ion is produced, this is an acidic process, but since disinfecting chlorine is a weak acid, only some of it breaks down in a way that lowers pH, as shown above (i.e. only HOCl produces H+; OCl- does not). During the process of chlorine breakdown by sunlight, there are hydroxyl (OH•), oxygen anion (O-•) and chlorine (Cl•) radicals that are also produced as short-lived intermediates (technical details in this post Pool Water Chemistry). This can help oxidize organics in the pool.
Net Chlorine To Breakpoint (Ammonia "Oxidation")
2NH3 + 3HOCl → N2(g) + 3H+ + 3Cl- + 3H2O
OCl- + H+ → HOCl
The disinfecting form of chlorine (HOCl) combines with ammonia through a series of reactions (that are not shown), with the net result being the production of nitrogen gas plus hydrogen ion and chloride ion. Though by itself this would be a strong acid reaction, there is also OCl- present that will combine with hydrogen ion to form more HOCl since the ratio of HOCl to OCl- will remain constant (and is about 50/50 at pH 7.5). Therefore, the net reaction is acidic but not strongly so. Further technical details are in this post.
The net reactions are as follows if you combine the ones shown above.
2NaOCl → 2Na+ + 2Cl- + O2(g)
3NaOCl + 2NH3 → 3Na+ + 3Cl- + N2(g) + 3H2O
The overall net reaction of adding sodium hypochlorite to your pool and having it used up in its most typical ways is to produce salt (yes, sodium chloride or table salt, dissolved in water) and oxygen or nitrogen gas (and water). That is why using liquid chlorine in a pool will raise the salt level…a chlorine pool is a salt pool.
Other things that could happen
Suppose you do not have enough chlorine in your pool relative to your bather load (ammonia demand). In that case, the chlorine may not completely oxidize ammonia, and instead, you will get chloramines (first, monochloramine). This reaction is basic. However, sunlight (UV) may break down monochloramine, which will result in the rest of the breakpoint process, which overall is acidic (same as shown above)
It is also possible for chlorine to combine with organic compounds to form chlorinated organics that are hard to break down. When people talk about the health problems with chlorine, it is usually about some of these chlorinated organics (Disinfection By-Products, DBPs) known as Tri-Halo-Methanes(THMs) such as chloroform. Also, some chloramines such as nitrogen trichloride (NCl3) not only smells foul but can cause health problems (especially in indoor pools with poor air circulation). In an outdoor pool exposed to sunlight and with a good chlorine residual, you typically don't get these "bad" compounds.
Salt (SWG) Pool
In a saltwater pool, you produce chlorine through the following reactions:
At the anode (positive plate): 2Cl- → Cl2(g) + 2e-
At the cathode (negative plate): 2H2O + 2e- → H2(g) + 2OH-
This nets out to the following where the chlorine gas dissolves in water:
2H2O + 2Cl-(aq) electricity→ Cl2(g) + H2(g) + 2OH-(aq)
The chlorine gas reacts with water to produce hypochlorous acid:
Cl2(g) + H2O → HOCl(aq) + H+(aq) + Cl-(aq)
Some of the hypochlorous acid will disassociate to form hypochlorite (dependent on pH):
HOCl(aq) ⇌ H+(aq) + OCl-(aq)
A small amount of water will stay in equilibrium with both proton and hydroxide ions:
H2O ⇌ H+(aq) + OH-(aq)
The net reactions are:
2H2O + Cl- → HOCl + OH- + H2(g)
or equivalently
H2O + Cl- → OCl- + H2(g)
Note that the products of HOCl and OH- are exactly the same as you get when you add liquid chlorine or bleach (ignoring sodium ion). This process is partly basic, but not strongly so due to the HOCl weak acid. Therefore, the overall net result in a salt pool is simply the production of oxygen or nitrogen gases. The disinfecting chlorine that was created from chloride ion gets converted back to chloride ion as it is used to sanitize.
The net reactions in an SWG pool for chlorine addition from the SWG and then breakdown from sunlight and oxidation of ammonia are as follows:
2H2O → O2(g) + 2H2(g)
2NH3 → N2(g) + 3H2(g)
The chlorine is not "seen" in the above net reactions because the chloride that became chlorine goes back to being chloride again. The oxygen gas comes from water when chlorine gas dissolved in it (i.e. from hypochlorite ion or hypochlorous acid) while the nitrogen gas comes from the ammonia (the oxygen or hydroxyl in the chlorine reverts back into water in this case, using the hydrogen from the ammonia to do so).
SWG should be installed in a proper fashion according to manufacturer specification. When they are installed properly, the vast majority of the chlorine dissolves. If not installed properly, there is always a slight chance of Cl2 venting to the atmosphere. And when it is vented, the Cl2 that is produced in a SWG is not converted to HOCl and HCl, which changes the balance of H+ to OH- in favor of the hydroxide ion which will cause an increases in pH.
pH and TA Adjustments
Lowering pH by adding Muriatic Acid
HCl (aq) → H+ (aq) + Cl- (aq)
When adding muriatic acid (hydrochloric acid, HCl) to a swimming pool, the aqueous (aq) acid dissociates into hydrogen ions (H+) and chloride ions (Cl-) when mixed with water, lowering the pH of the pool water.
pH = -log10[H+]
pH is the negative log base 10 of hydrogen ion activity. Therefore, when you add H+ (Hydrogen ions), you lower pH, as pH is a measure of hydrogen ion activity. Adding muriatic acid also adds Cl-, which is, chloride ions…exactly how salt is measured in a pool. So when you add muriatic acid, you are adding salt.
How Muriatic acid lowers Total Alkalinity (TA)
HCl → H+ + Cl−
H+ + CO32- → HCO3-
H+ + HCO3 → H2CO3
H2CO3 → H2O + CO2(g)
Muriatic acid, when added to the pool, completely dissociates into Hydrogen and Chloride ions. The Hydrogen ions (H+) will react with carbonate ions (CO32-), if they are present in the pool, converting them into bicarbonate ions (HCO3−). The bicarbonate ions then react with more hydrogen ions from the acid to form carbonic acid (H2CO3). Carbonic acid is unstable and breaks down into water (H2O) and carbon dioxide (CO2). Total alkalinity is lowered as Carbonic acid breaks down. Total alkalinity is a measure of the total concentration of alkaline substances (like carbonates and bicarbonates) in the pool water.
Natural pH rise
CO2 + H2 ⇌ H+ + HCO3-
Equilibrium of Carbon dioxide, Hydrogen ions and Carbonic acid in the pool. When CO2 outgasses the equilibrium shifts left, reducing the amount of Hydrogen ions resulting in pH rise. For more details on why CO2 outgasses, see PH TA Relationship.
Raising pH with 20 mule team borax
Dissolution of Borax:
Na2B4O7⋅10H2O → 2Na+ + B4O72− + 10H2O
Hydrolysis of Tetraborate Ions:
B4O72− + 7H2O → 4H3BO3 + 2OH−
Net Reaction:
Na2B4O7⋅10H2O + H2O → 2Na+ + 4H3BO3 + 2OH−
20 Mule team Borax is the sodium tetraborate decahydrate (Na2B4O7 · 10H2O) that, when dissolved in water, is hydrolyzed to Sodium ions, boric acid and Hydroxide ions (OH−). The converse of H+ ions in measuring pH are OH- ions. A higher concentration of OH- anions results in a higher pH.
Raising Total Alkalinity with Baking Soda
Dissolution of Baking Soda:
NaHCO3 + H2O → Na+ + HCO3-(aq)
Reaction of Bicarbonate Ion with Water:
HCO3− + H2O ⇌ H2CO3 + OH-
Overall Reaction
NaHCO3 + H2O ⇌ Na+ + OH− + H2CO3
When baking soda (sodium bicarbonate, NaHCO3) is added to water, it dissolves, and results in a rise in sodium ions (Na+), Hydroxide ions (OH-) which raises pH, and H2CO3 which raises Total Alkalinity. As a result, baking soda raises TA and pH.
Calcium Adjustments
Raising CH with Calcium Chloride
CaCl2(s) → Ca2+(aq) + 2Cl−(aq)
Calcium hardness is a measure of the concentration of calcium ions (Ca2+) in water. Increasing Ca2+ will raise calcium hardness (CH).
Lowering CH
There is no way to reduce CH except replacing water, or using reverse osmosis.
Cyanuric Acid (CYA)
To simplify the discussion, we will use the dominate species of H2CY- cyanuric acid and the strongly disinfecting and oxidizing form of chlorine, HOCl Hypochlorous acid. OCl- will act the same way as HOCl. There are many species of cyanuric acid that exist at various pKa. See O'Brien in the references for a deeper dive into the cyanuric acid chlorine complexes.
Dissolution of Cyanuric Acid in Water
CyA(s) → CyA(aq)
CyA(aq) ⇌ H+ + H2CY-(aq)
Cyanuric acid (CyA), commonly just referred to as CYA, has the chemical formula C3H3N3O3 or (CHNO)3 and can also be referred to as CyA or H3Cy where the Cy refers to the (CNO)3 cyclic structure. When dissolved in swimming pool water it exists as the weak acid and the first disassociated cyanurate ion (H2CY-) where the pKa is given as 6.88. At typical pool water pH levels CYA is mostly present as the cyanurate ion until it reacts with free chlorine. CYA testing measures both the acid and the cyanurate ion.
Interaction with Chlorine
HOCl + H2CY- ⇌ HClCy- + H2O
Cyanuric acid primarily acts to stabilize free chlorine in pool water. This stabilizing effect is achieved through the formation of a cyanuric acid chlorine complex. This complex slows down the degradation of chlorine by UV light, extending its effectiveness as a disinfectant. The reaction is in equilibrium, meaning the chlorine can still be used for sanitation, but it is protected from photodegradation, until the HOCl is used on the left side of the equation, freeing more HOCl from the cyanuric acid chlorine complex.
Cyanuric Acid as a Weak Acid
Though cyanuric acid is a weak acid, it does not significantly lower the pH of pool water when added in typical concentrations. It has a pKa around 4.0, which means that it only slightly dissociates to produce hydrogen ions (H+) in water, lowering pH. This dissociation is minimal, and its primary role is as a chlorine stabilizer rather than as an acid that significantly impacts water chemistry.
Salt
Increasing salt
NaCl(s) → Na+(aq) + Cl−(aq)
When sodium chloride (NaCl) dissolves in water, the polar water molecules surround the individual Na+ and Cl− ions, causing them to separate and disperse evenly in the solution. The salt simply dissociates, and no new chemical bonds are formed or broken. Cl- is the primary measure used in salt tests for pool water.
Decreasing salt
The only way to remove salt is to replace water or use reverse osmosis.
Boric Acid
Boric Acid dissolved in water
H3BO3(s) → H3BO3(aq)
At normal pool pH, most boric acid stays as H₃BO₃, with some converting to B(OH)₄⁻
Weak acid equilibrium (buffering):
H3BO3 + H2O ⇌ B(OH)4- + H+
This creates a buffering system that resists upward pH drift.
Boric acid reducing pH rise in a salt cell
At the cathode (negative plate): 2H2O + 2e- → H2(g) + 2OH-
This increases OH⁻, which raises the local pH inside the salt cell.
As indicated earlier, boric acid participates in the buffering equation.
H3BO3 + H2O ⇌ B(OH)4- + H+
When OH⁻ builds up (like in a salt cell), boric acid reacts with that OH⁻ to form borate. This can reduce the potential for scaling within the cell.
H3BO3 + OH- → B(OH)4-