We have a newer inground pebble Gunite pool that just got finished in September. Our pool builder was out two days ago finishing up punch out items and finally took a look at some staining that had been on the plaster since start up. I tested the water today and the pH was 6.2! I’ve been perfectly maintaining the chemistry and now they came and jacked it all up. I don’t know what specifically the pool builder did, they didn’t contact us at all, but I imagine they added a bunch of acid. We have been using our heater this whole time and I’m very concerned that this low pH will damage our heater. And of course we won’t know for sure until down the road when our PB is off the hook. Will 2 days of using the heater with low ph cause long term damage?
PH 6.2- help!
- Thread starter katiejean13
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- Jun 24, 2021
- 16,388
- Pool Size
- 29000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60 Plus
Do you have pool math (Link-->PoolMath)
Adjust pH first. You can use 20 mule team borax, or Soda Ash/Washing Soda. This will raise your pH and also your TA slightly as well. Once you've added, recheck pH and TA (say 30-60 minutes with pump running). It may be a good idea to less than the full amount right away. It's easy to add more, hard to add less after the fact. Confirm amounts with Pool Math!
Then if your TA still needs to be raised (it likely will), you can use baking soda to raise TA. Use pool math to help you and your new TA reading as the starting point. As long as your TA is above 50 ppm, I'd see how your pH behaves and don't intentionally raise it any higher than 60 unless you observe erratic pH "bounce" at that TA level.
Yep, that’s what I use to track my tests. Thank you. I’ll give that a try. Any idea if a few days of low PH will damage our heater? We basically have it running whenever the pump runs to maintain temp. And had no idea the ph was that low for a couple days.
- Jun 24, 2021
- 16,388
- Pool Size
- 29000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-60 Plus
*Probably not* A few days at 6.8 isn't horrible. If, in fact, it is 6.8. Unless you have calibrated pH meter, you really don't know how low it is if you are using the Taylor chems.Any idea if a few days of low PH will damage our heater?
I wouldn't worry about it, lets get that water fixed!
- Jan 7, 2012
- 2,128
- Pool Size
- 21000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
With a TA of 10, they probably added a bunch of acid and the pH was probably in the 5.0 range, which can strip the copper from the heat exchanger.
The copper actually develops a copper oxide layer that protects it from oxidation.
Low pH strips the copper oxide layer and exposes the elemental copper metal to oxidizers like chlorine and oxygen.
The copper oxide needs to reform to protect the heat exchanger from further oxidation damage.
In my opinion, it is always better to use baking soda over soda ash as soda ash can cause unwanted side effects.
In my opinion, I would never use soda ash (sodium carbonate) for any reason.
If you want borates, you can use Borax instead of baking soda.
The copper actually develops a copper oxide layer that protects it from oxidation.
Low pH strips the copper oxide layer and exposes the elemental copper metal to oxidizers like chlorine and oxygen.
The copper oxide needs to reform to protect the heat exchanger from further oxidation damage.
In my opinion, it is always better to use baking soda over soda ash as soda ash can cause unwanted side effects.
In my opinion, I would never use soda ash (sodium carbonate) for any reason.
If you want borates, you can use Borax instead of baking soda.
Yup... that's a Florida filter 
Katiejean, did you register your plaster finish when it was done? Is that NPT? You can find the registrations for these fancy finishes online.
Maddie
Katiejean, did you register your plaster finish when it was done? Is that NPT? You can find the registrations for these fancy finishes online.
Maddie
The goal in either case is to pick up hydrogen to increase the pH.
If all carbonate ions picked up 2 hydrogen, then it only raises the pH.
The problem is that the carbonate ions also combine with calcium and this can be a problem.
Na2CO3 --> 2Na+ + CO32- + Ca2+ --> 2Na+ + CaCO3
Na2CO3 --> 2Na+ + CO32- + 2H+ --> 2Na+ + H2O + CO2
NaHCO3 --> Na+ + HCO3- + H+ --> Na+ + H2O + CO2
Sodium carbonate creates high levels of carbonate, which can combine with calcium and precipitate out as calcium carbonate.
In some cases, it can make a big mess and create cloudiness and scale.
Sodium bicarbonate avoids problems because bicarbonate does not precipitate out with calcium.
The end result is the same either way with the production of carbon dioxide, so there is no benefit to using sodium carbonate over sodium bicarbonate only risk of cloudiness and scale.
If all carbonate ions picked up 2 hydrogen, then it only raises the pH.
The problem is that the carbonate ions also combine with calcium and this can be a problem.
Na2CO3 --> 2Na+ + CO32- + Ca2+ --> 2Na+ + CaCO3
Na2CO3 --> 2Na+ + CO32- + 2H+ --> 2Na+ + H2O + CO2
NaHCO3 --> Na+ + HCO3- + H+ --> Na+ + H2O + CO2
Sodium carbonate creates high levels of carbonate, which can combine with calcium and precipitate out as calcium carbonate.
In some cases, it can make a big mess and create cloudiness and scale.
Sodium bicarbonate avoids problems because bicarbonate does not precipitate out with calcium.
The end result is the same either way with the production of carbon dioxide, so there is no benefit to using sodium carbonate over sodium bicarbonate only risk of cloudiness and scale.
Note: You only need baking soda.
Most people believe that baking soda (sodium bicarbonate) does not raise the pH very much.
However, that is only true when the pH is close to 8.3.
The farther the pH is from 8.3, the more effect baking soda has on the pH.
As the pH gets closer to 8.3, the effect on the pH slows down exponentially and stops at 8.3.
When the pH starts out very low, like 4.5 to 5.0, baking soda actually raises the pH quite a bit.
If you raise the TA with baking soda, the pH will immediately get to between 6.3 and 7.0 in most cases, which is out of the danger zone.
This process creates a lot of carbon dioxide and the carbon dioxide offgasses over the next 12 hours and the pH should be about 7.2 to 7.4 in the morning of the next day.
This is a good safety mechanism because you can’t overshoot the pH beyond 8.3 regardless of how much baking soda you use.
In fact, if the pH was over 8.3, baking soda would lower the pH.
Sodium carbonate, however, can overshoot the pH to 10.3, which can be a huge problem.
Most people believe that baking soda (sodium bicarbonate) does not raise the pH very much.
However, that is only true when the pH is close to 8.3.
The farther the pH is from 8.3, the more effect baking soda has on the pH.
As the pH gets closer to 8.3, the effect on the pH slows down exponentially and stops at 8.3.
When the pH starts out very low, like 4.5 to 5.0, baking soda actually raises the pH quite a bit.
If you raise the TA with baking soda, the pH will immediately get to between 6.3 and 7.0 in most cases, which is out of the danger zone.
This process creates a lot of carbon dioxide and the carbon dioxide offgasses over the next 12 hours and the pH should be about 7.2 to 7.4 in the morning of the next day.
This is a good safety mechanism because you can’t overshoot the pH beyond 8.3 regardless of how much baking soda you use.
In fact, if the pH was over 8.3, baking soda would lower the pH.
Sodium carbonate, however, can overshoot the pH to 10.3, which can be a huge problem.
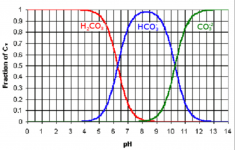
When the pH is really low, the equilibrium forces most of the bicarbonate to become carbonic acid or aqueous carbon dioxide.
So, the bicarbonate picks up a hydrogen to become carbonic acid and this raises the pH by removing hydrogen ions.
NaHCO3 --> Na+ + HCO3- + H+ --> Na+ + H2O + CO2
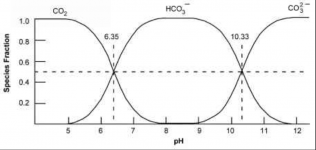
As the pH rises, the percentage that is bicarbonate vs. carbonic acid changes and the molar percentages at a pH of 6.35 are equal at 50% each.
So, added bicarbonate at first converts almost totally to carbonic acid and then by a pH of 6.35 only half converts to carbonic acid and the pH rise begins to slow.
When the pH gets to 8.3 all bicarbonate stays as bicarbonate and the pH does not change.
If the pH was over 8.3, the bicarbonate would act like and acid by releasing a hydrogen ion to become carbonate and thus lowering the pH.
For example, if the pH was 10.33, half of the bicarbonate would become carbonate and the pH would go down.
So, bicarbonate always pushes the pH towards 8.3 and the further the pH is from 8.3, the stronger the effect is on the pH.

So, the bicarbonate picks up a hydrogen to become carbonic acid and this raises the pH by removing hydrogen ions.
NaHCO3 --> Na+ + HCO3- + H+ --> Na+ + H2O + CO2
As the pH rises, the percentage that is bicarbonate vs. carbonic acid changes and the molar percentages at a pH of 6.35 are equal at 50% each.
So, added bicarbonate at first converts almost totally to carbonic acid and then by a pH of 6.35 only half converts to carbonic acid and the pH rise begins to slow.
When the pH gets to 8.3 all bicarbonate stays as bicarbonate and the pH does not change.
If the pH was over 8.3, the bicarbonate would act like and acid by releasing a hydrogen ion to become carbonate and thus lowering the pH.
For example, if the pH was 10.33, half of the bicarbonate would become carbonate and the pH would go down.
So, bicarbonate always pushes the pH towards 8.3 and the further the pH is from 8.3, the stronger the effect is on the pH.




Last edited:
The uncomplicated advice from TFP is to use baking soda (not soda ash) to raise your TA to around 60 ppm or so.
Once that's done, retest your pH and do what's necessary to bring it into the 7's.
I doubt there is ANY harm to your equipment for that short low pH exposure. Even if there is a little affect, how in the world could you tell?
Once that's done, retest your pH and do what's necessary to bring it into the 7's.
I doubt there is ANY harm to your equipment for that short low pH exposure. Even if there is a little affect, how in the world could you tell?
BMerrill
Gold Supporter
- Mar 8, 2021
- 380
- Pool Size
- 17860
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Turbo Cell (T-CELL-5)
pH of drinkable Orange Juice is somewhere below 4.0, I think. Pool water of 6.2 will have little affect on people.
Bring it up into the 7's as TFP suggests and go swimming,
Bring it up into the 7's as TFP suggests and go swimming,
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.


