- May 3, 2007
- 17,281
- Pool Size
- 20000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
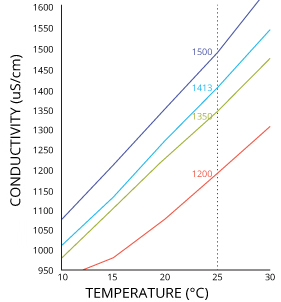
Technically the electrolytic reaction is proportional to current due to the electron reaction with the chloride ions. But in reality given a certain cell design, it turns out to be both current and power when voltage and conductance are fixed.
Chlorine production - Wikipedia
Chlorine production - Wikipedia