Here are my measurements from my last visit...
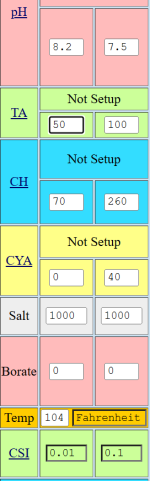
8+
190 TA
350 CH
0 CYA
3200 Salt/TDS
Inground spa size is about 1400 gallons.
I adjusted pH down to 7.2 and the alkalinity came down to 170.
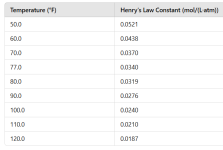
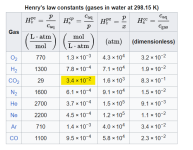
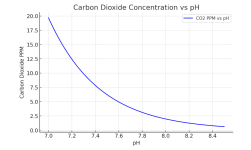
Customer has naturally high alkalinity in the water. Upon filling the spa a couple months ago, the alkalinity was well over 250 as I recall. He rarely uses it and keeps the pump running 24/7 right now in the winter with temperature set to 71 unless he's using it, then heats up to 100+. Pool also remains covered most of the time with vinyl cover (not insulated). I just started going there on a regular basis but everytime I show up I have had to add 2+ cups of acid to bring the pH down. The alkalinity has then been slowly dropping with each visit. Is the running pump just increasing the alkalinity through aeration? Even though there isn't really visible air/aeration happening.
8+
190 TA
350 CH
0 CYA
3200 Salt/TDS
Inground spa size is about 1400 gallons.
I adjusted pH down to 7.2 and the alkalinity came down to 170.
Customer has naturally high alkalinity in the water. Upon filling the spa a couple months ago, the alkalinity was well over 250 as I recall. He rarely uses it and keeps the pump running 24/7 right now in the winter with temperature set to 71 unless he's using it, then heats up to 100+. Pool also remains covered most of the time with vinyl cover (not insulated). I just started going there on a regular basis but everytime I show up I have had to add 2+ cups of acid to bring the pH down. The alkalinity has then been slowly dropping with each visit. Is the running pump just increasing the alkalinity through aeration? Even though there isn't really visible air/aeration happening.