It because of the chemistry of how the DPD dye works and what the FAS reagent does. I'll try to put in simple terms so as to not have to revert to a lot of diagrams or chemical formulae.
The DPD indicator has three forms - an unoxidized amine form, an oxidized Würster configuration and an additional oxidized form as an imine structure. This is what happens to DPD in the presence of an oxidizer like chlorine -
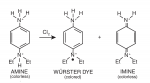
(sorry for the tiny pic)
The colorless reduced form (amine) reacts with chlorine and forms the pink colored Würster configuration. There is also a very small amount of oxidized imine created which is dependent on pH and the concentration of the oxidizer and this is the origin of DPD indicator getting bleached out to clear when the chlorine is too high. At normal testing pH and FC levels, the amount of imine created is not worth worrying about. Combined chlorine compounds, which for the most part should be monochloramine and a small amount of dichloramine, do not initially react with the DPD indicator and so none of the dye is oxidized to the pink color by the CC's. CC's are weak oxidizers but, given enough time, they can cause the DPD dye to turn pink which is the origin of "chloramine breakthrough" when someone lets the sample sit for too long and it starts to turn pink.
The FAS titrating reagent is ferrous ammonium sulfate and it is a reducing agent, that is it will make something that is oxidized become "un-oxidized" or reduced. In the case of the DPD indicator, when you add FAS titrating reagent you are converting the Würster compound from it's oxidized form (pink) back to its colorless amine form. So, by getting the concentrations of FAS right and making sure the volumes dispensed are accurate, the amount of FAS added is directly proportional to the amount of chlorine that oxidized the DPD in the first place.
So how are CC's measured?
The R-0003 reagent is nothing more than potassium iodide (KI). When you add iodide to an acidic, aqueous solution containing a weak oxidizer like monochloramine, the iodide (I
-) gets converted to the triiodide anion (I
3-). Triiodide is a strong enough oxidizer to react with the DPD amine dye and convert it into the pink, oxidized Würster dye. That reaction happens completely and then adding the FAS titrating reagent reduces the pink form to clear again. So now the second volume of FAS added is directly proportional to the amount of triiodide that caused the amine to turn pink which in turn is directly proportional to the original amount of CCs in the water sample.
So why doesn't FAS react with CC's?
Well, CC's are weak oxidizers and do not react quickly with the FAS reagent at low pH. Given enough time, and perhaps a rise in pH, then there would be some cross reaction of FAS with CC's. But, given how quickly the test is performed and the low pH of the sample after adding the R-0870 powder, there is no loss of CCs. A single, extra drop between the FC and CC parts of the test will have very little influence on the reaction of CC's with iodide and then iodide with the DPD dye. So, when you follow the protocol, the test is fairly accurate. And of course this is all based on the caveat that you are, to the best of your ability, following the test protocol and not intentionally trying to overdo it. Certainly if one added 10 extra drops of FAS titrating reagent beyond the pink/clear endpoint and then let it sit for a few minutes before doing the CC test, you would expect inaccurate results.


