- May 31, 2023
- 27
- Pool Size
- 19400
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Background: Mostly curiosity in the science with this question. Learning as much as possible helps things stick better when I'm learning something new. 5th season with our pool, but I took ownership of caring for it just this year. And this group is what finally got me to where I am only spending 15-30 minutes on my pool each day, rather than hours scrubbing, testing, brushing, crying, sweating, yada, yada!
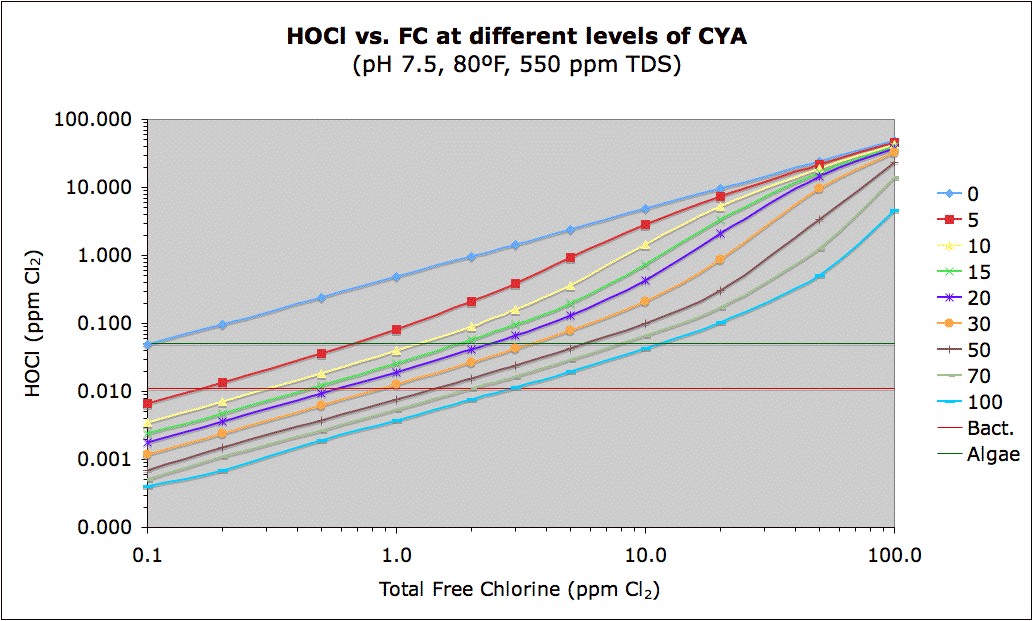
Can someone explain, in layman's terms, why the "industry standard" for FC levels is always 1-4ppm when everything I read from actual owners & non-industry experts says it is based on CYA levels? Even my hayward aquarite says 1-3ppm inside the power box on the sticker with all of the various directions. Their recommended CYA levels are 60-80, yet I've learned it should actually be a percentage of my CYA. Even at the minimum CYA of 60, that would make my ideal FC level 4.5ppm (I believe...based on 7.5%, though that's just off the top of my head so I could be slightly off with that actual percentage...i did not go back & actually look up that # since I use the pool math app for ideal level #s).
And then there are the other questions that come into play with "shocking" & "superchlorinating". Using the pool math app & TFP methods, what gives me that shock level #? You can't find anything out there, industry-wise, that says "to shock your pool, you should bring your FC levels to XXppm" except a few sources here & there that say it should be your CC x10 (I think) or something to that effect.
Questions:
1. Why the 1-3/4 ppm recommendation even by the companies that are not out to sell you chemicals? (i.e. Hayward & other salt cell manufacturers)
2. How is FC shock level calculated?
3. Why is my ideal TA so much lower with a salt pool? (I understand what the TA does as a pH buffer, but don't really understand the science behind it being a lower recommended # than industry standard. Same with CH really, except that recommendation seems to be high than industry standard)
4. With some of my higher FC "encapsulated" by stabilizer, does that really mean it's just sitting there, floating along until it's called up for duty, lol? If the excess (above what's getting used, at any given moment, to actually sanitize) is "locked up", how does it get released when it's needed?
Not looking to read more articles about chemistry, as I've read a TON. These questions are coming after reading all of those articles. Anyway, just curious mostly. No issues at the moment, (finally got the black algae under control after learning that the biggest culprit in keeping chlorine levels stable, after killing the algae, was actually an issue with our aquarite system not making chlorine AT ALL, and this forum's "more about..." links helped me realize it was not even making chlorine & pinpoint what was broken. I FINALLY got past the OCLT!) just looking for an opportunity to learn more so I have to ask less and can help others more effectively!
I can't thank the group here enough for getting me where I am today. Y'all have been AMAZING!
Can someone explain, in layman's terms, why the "industry standard" for FC levels is always 1-4ppm when everything I read from actual owners & non-industry experts says it is based on CYA levels? Even my hayward aquarite says 1-3ppm inside the power box on the sticker with all of the various directions. Their recommended CYA levels are 60-80, yet I've learned it should actually be a percentage of my CYA. Even at the minimum CYA of 60, that would make my ideal FC level 4.5ppm (I believe...based on 7.5%, though that's just off the top of my head so I could be slightly off with that actual percentage...i did not go back & actually look up that # since I use the pool math app for ideal level #s).
And then there are the other questions that come into play with "shocking" & "superchlorinating". Using the pool math app & TFP methods, what gives me that shock level #? You can't find anything out there, industry-wise, that says "to shock your pool, you should bring your FC levels to XXppm" except a few sources here & there that say it should be your CC x10 (I think) or something to that effect.
Questions:
1. Why the 1-3/4 ppm recommendation even by the companies that are not out to sell you chemicals? (i.e. Hayward & other salt cell manufacturers)
2. How is FC shock level calculated?
3. Why is my ideal TA so much lower with a salt pool? (I understand what the TA does as a pH buffer, but don't really understand the science behind it being a lower recommended # than industry standard. Same with CH really, except that recommendation seems to be high than industry standard)
4. With some of my higher FC "encapsulated" by stabilizer, does that really mean it's just sitting there, floating along until it's called up for duty, lol? If the excess (above what's getting used, at any given moment, to actually sanitize) is "locked up", how does it get released when it's needed?
Not looking to read more articles about chemistry, as I've read a TON. These questions are coming after reading all of those articles. Anyway, just curious mostly. No issues at the moment, (finally got the black algae under control after learning that the biggest culprit in keeping chlorine levels stable, after killing the algae, was actually an issue with our aquarite system not making chlorine AT ALL, and this forum's "more about..." links helped me realize it was not even making chlorine & pinpoint what was broken. I FINALLY got past the OCLT!) just looking for an opportunity to learn more so I have to ask less and can help others more effectively!
I can't thank the group here enough for getting me where I am today. Y'all have been AMAZING!