I agree that there will be "some" mixing. However, there are some aspects to the flow within the cell which may minimize the mixing. In the middle area between plates, there is definitely turbulence under typical flow rates. However, velocity always goes to zero at a stationary boundary (i.e. the plates). Slightly off the plates, there is laminar flow (i.e. non-turbulent) and only in the middle area is there turbulent flow. But turbulent flow does not always result in macro mixing. In smooth pipes and ducts, it is generally a very localized phenomenon and the eddies tend to be very small.
I did a CFD simulation of a pair of plates with an stepped entrance to simulate the plastic holder. The inlet has a uniform velocity of 0.5 m/s along the X-axis over the entire inlet equivalent to ~35 GPM through the cell. I used two transport injection points on opposite plates near the inlet to show how particles would get carried out into the flow and mix together. Of course this is happening all over the plate surfaces but by using only two points near the inlet, it is easier to visualize.
The simulation uses a steady state RANS model implemented by OpenFoam to solve the Navier–Stokes equations. Here is some background on OpenFoam & RANS for anyone not familiar with them:

en.wikipedia.org

en.wikipedia.org
The advantage of using RANS over DNS (direct numerical simulation) is a much larger mesh size can be used at the expense of resolution (i.e. internal cell flow) but not accuracy. I used .2 mm cell spacing which resulted in over 300k cells and 3 hours of run time. Steady state solver residual convergence was better than 3E-4 so the results should be fairly accurate.
CFD Results
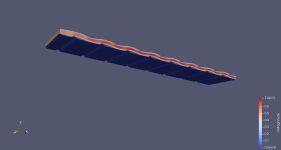
This plot shows the velocity magnitude between the cell plates. X direction is the direction of flow through the cell (150mm), Y direction is the width of the cell (62mm) and the Z direction is the gap separation between the plates (4mm).
View attachment 602547
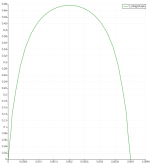
Here is the cross-sectional velocity profile along the Z-axis at the cell X-axis mid point.
View attachment 602548
Note that the velocity goes to zero at the walls.
Here is Uz, the velocity along the Z-axis. This is the velocity component which would move the particles generated on each plate toward each other.
View attachment 602552
Most of the area is very close to zero. Only near the inlet is there an elevated Uz component. These are small eddies that occur due to the step in plate separation near the inlet.
Here is the cross sectional view of Uz at the X-axis mid point:
View attachment 602549
Note that this velocity is quite small so very little mixing would occur in this location.
Here is the same two cross sectional plots but closer to the inlet side along the X-axis.
View attachment 602557
Again, velocity goes to zero at the walls. The velocity has more of a flat top here again because of the inlet transition.
View attachment 602558
Uz is higher in this location so a bit more mixing should be expected near the inlet but tapers off away from the inlet.
Here are two plots which show the two transport injection concentrations and where mixing might occur.
X-axis mid point along Z-axis:
View attachment 602553
This would imply that the concentration ratio at each plate is weighted very heavily to the elements generate on that plate.
X-axis inlet:
View attachment 602563
The inlet is closer to the injection point which is why the concentration is much higher at the walls but even with the larger Uz velocity component, not much mixing occurs here since it is close to the injection point.
So while there is mixing going on, it just doesn't appear to be very much, especially near each plate. However, I would expect mixing to increase with the amount of scale on the plates as this would cause additional eddies to occur along the length of the plates. I believe that OpenFoam has the ability to model surface roughness so I may look into that to see how much of a difference it makes.
So in summary, I am not suggesting that the elements generated at each plate remain fully separated throughout the entire cell. I am only suggesting that perhaps the concentrations remain mostly weighted to the side of generation and therefore, PH neutralization due to mixing may not be that significant.