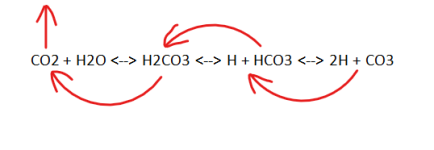If you want to avoid pH rise in a hot tub, you probably need to be in the range of pH = 7.8 to 8.0 with a carbonate alkalinity of 30 to 50 ppm.
30 ppm Carbonate Alkalinity gives you very little room for error because any acid added can crash the pH fast.
For example, in a 700 gallon Spa/Hot Tub, 1 oz acid will lower the TA by 5.6 ppm.
5.35 oz acid will lower the TA by 30 ppm in a 700 gallon Spa/Hot Tub.
At 30 ppm carbonate alkalinity, you should not use trichlor tabs as they can and will crash the pH and alkalinity quickly and destroy the heater.

30 ppm Carbonate Alkalinity gives you very little room for error because any acid added can crash the pH fast.
For example, in a 700 gallon Spa/Hot Tub, 1 oz acid will lower the TA by 5.6 ppm.
5.35 oz acid will lower the TA by 30 ppm in a 700 gallon Spa/Hot Tub.
At 30 ppm carbonate alkalinity, you should not use trichlor tabs as they can and will crash the pH and alkalinity quickly and destroy the heater.