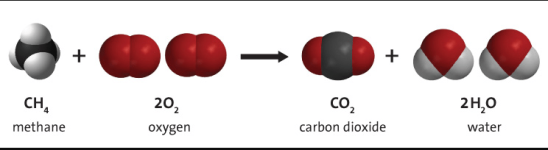
“Swimming pools first appeared in the NEC in the 1962 edition, and the section was a brief three pages long.
Here’s the first bonding citation in the 1962 code, although it was not initially called bonding:
680-7. Grounding
- (a) All metallic conduit, piping systems, pool reinforcing steel, light fixtures, and the like, shall be bonded together and grounded to a common ground.
The metal parts of ladders, diving boards, and their support, shall be grounded.
- (b) No pool equipment shall be grounded to an external grounding electrode that is not common to the pool ground (deck box or transformer enclosure).
- (c) An unbroken No. 14 AWG, or larger, insulated copper wire shall be provided for a grounding conductor from the deck box to the distribution panel ground.
- (d) Metallic raceways shall not be depended upon for grounding.
Where exposed to pool water and in other corrosive areas such as in pump houses or adjacent to water treating and other equipment, the grounding of the non-current carrying parts shall be by means of an insulated copper conductor sized in accordance with Section 250-95.
The 1965 edition changed the ground wire requirement from #14 AWG to the larger #8 AWG, along with other minor additions.
Then the 1968 edition introduced the word “bonding” for the unbroken connection between all components that was required, and specified the five-foot radius rule that we know today.
Equipotential bonding code has continued to be improved in each code cycle up to today.
While the year that the NEC added a new requirement is easy to define, each local jurisdiction’s building codes don’t necessarily adopt the latest edition of the NEC immediately.
The state of Florida, for example, did not make the 2011 NEC effective until mid-2015.
Other jurisdictions have sometimes waited even longer to adopt a newer NEC edition and, to complicate things further; they might make amendments that exclude parts of the newest requirements.
So the year when the NEC first adds or changes a requirement can be several years before your local building department adopted that edition of the code and began enforcing it”.
www.howtolookatahouse.com