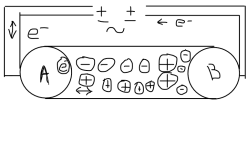
If the voltage was DC, then you would get a buildup of charge at the plates and the current would stop flowing unless you had electrolysis.
With AC, the voltage changes polarity based on the frequency.
Basically, the electrons go right up to the water, but they do not actually go into the water.
The ions in the water go right up to the sensor plates.
So, the ions and electrons are as close to each other as the electrons in the wire are to each other.
So, the water between the plates is the conductor and it acts just like a metal wire where the ions are acting like electrons to carry the charge and the energy due to the alternating electric and magnetic field.
Even in a wire for AC, the electrons do not move that much.
They basically oscillate back and forth as the electric and magnetic fields alternate and the current, charge and energy is carried along.
Part of the problem when talking about things like electrons, current, voltage, power, energy and fields is that we try to represent quantum processes in macroscopic terms when there are no accurate macroscopic term that apply.
We talk about the particle or wavefunction, but these are just crude approximations of something that we do not fully understand.