- Mar 5, 2020
- 3,221
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
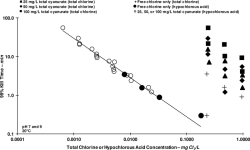
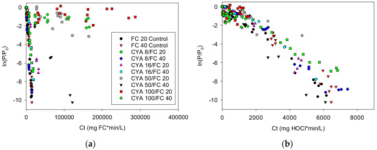
The equilibrium is skewed heavily enough to reverse the reaction to HOCL production and exhaust the FC within the same time frame as water with little to no CYA (with only demand as a catalyst).
What’s the equilibrium constant between species? I get that it’s dynamic, but it’s gotta have something that induces a move/change/delta and also solves for an increase in CT and oxidation/sanitation effectiveness.
There's nothing skewed, it's all just plain old equilibriums. The more CYA, the higher the proportion of chlorinated Cyanurates and the less HOCl, therefore higher total FC required to have sufficient HOCl to do the work. If some HOCl gets "used" by killing or oxidising stuff or by UV, then the proportions of HOCl and chlorinated Cyanurates reshuffle very quickly to the new FC. I find the term "release" of HOCl a bit oversimplified and it can be misleading.
It's not just one equilibrium, there are a couple of them which lead to a system of equations that need simultaneous solving which requires numerical methods.
You can find the complete set of equilibrium reactions/equations together with the equilibrium constants in O'Brien's paper:
Chem Geek had put together a spreadsheet that he used to solve the involved equations and to do all the calculations he is referring to in his sticky threads in the Deep End (I highly recommend to go through them). You can find the spreadsheet here:
I’m curious about less UV causing a stacking effect. I’m wondering if less UV not only reduces less FC but also retards the reaction between chlorine and CYA? In other words, is it a photo-reactive reaction?
I was more interested in the photo-reactivity of the reaction. Is UV not a contributing factor? It may not be and the shielding effect is purely a positive side-effect.
UV contributes in the sense that it leads to a quick decay of OCl- into Cl-, but the chlorinated Cyanurates are well protected from this decay. The equilibriums between CYA, HOCl and OCl- themselves are not UV dependent. But it influences the system by "removing" OCl- (and also some HOCl, but the UV induced HOCl decay is much slower than OCl- decay, so most of the HOCl gets lost via the indirect path of OCl- being lost and some HOCl then turning into OCl- to re-establish the equilibrium between the two, which is then susceptible to UV decay).
Experience has shown that the higher the CYA, the better the UV protection effect at identical FC/CYA ratios (which would result in identical HOCl and OCl- levels). Why this is so, we haven't fully understood. Chem Geek discussed this extensively in his chemistry thread. But that's why we recommend higher CYA levels with SWGs to make use of this effect while being at low risk of having to SLAM. We don't recommend these higher CYA levels for liquid chlorine pools, because the risk of having to SLAM is higher. And SLAMing at CYA 80 usually requires draining to reduce CYA first.
Some energy is released from the system as nitrogen and some chloride reforms with sodium to return to salt. The process repeats.
There is no recombination of Sodium and Chloride in water happening. When NaCl dissolves in water it separates inti Na+ and Cl- ions, and they remain separated. When we measure "salt" in the pool, we technically only measure the Cl- content. This Cl- could come from various sources, like added NaCl-salt, added CaCl2-salt, added chlorine (via liquid, Di-/Trichlor or Cal-Hypo, the chlorine in all these sources all ends up eventually as Cl-) or muriatic acid (which is hydrochloric acid or HCl which dissociates completely into H+ and Cl- when added to water).
While in water, the Na+ and Cl- ions remain dissociated, the sodium ions are just hanging around, having no direct involvement in all the chlorine reactions going on.
With a SWG, the Cl- ions are eventually "recycled" into FC, but otherwise the Cl- ions also just hang around with no further direct involvement in the reactions.