I have a Pentair cell. The instructions say 1 gallon water to 16 ounces muriatic acid and not to soak it for over 30 minutes. I have soaked it four times, up to forty-five minutes, and I am just starting to make progress. I even added extra acid this last time. Is this the best approach? Soaking longer is bad and more acid is bad? I rinse it and let it dry between soakings.
Calcium not coming off salt cell
- Thread starter Tinaja
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
- May 3, 2014
- 58,432
- Pool Size
- 6000
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
Your SWCG should not get scale build up if your pool water chemistry is properly managed.
You are destroying your cell.
Read SWG How It Works - Further Reading
You are destroying your cell.
Read SWG How It Works - Further Reading
- Jun 22, 2014
- 47,840
- Pool Size
- 17888
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-45 Plus
Have you tried using a small wooden stick to carefully try to knock off any larger chunks? I got a package of what looks like large tongue depressors from the arts and crafts section at Walmart. They are skinny enough to do between the blades of the cell.
- May 3, 2014
- 58,432
- Pool Size
- 6000
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
This sounds more like a non carbonate scale. If it does not violently react when you put acid on it, then it could be a calcium sulfate.
Do you use dry acid?
Do you use dry acid?
No, I don't use dry acid. Someone told me I am using the wrong shock, though. I was using calcium hypochlorite. I guess I need a new one. $$$This sounds more like a non carbonate scale. If it does not violently react when you put acid on it, then it could be a calcium sulfate.
Do you use dry acid?
Last edited:
- May 3, 2014
- 58,432
- Pool Size
- 6000
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
You have a SWCG, there should be no need for a 'shock'.
I assume you are using calcium hypochlorite.
Can you post a full set of test results?
I assume you are using calcium hypochlorite.
Can you post a full set of test results?
Since I had nothing to lose (since the salt cell was shot), I soaked it for hours with more acid, and there is still some calcium. So I think it is calcium sulfate. The long Texas summer, algaecide and the wrong shock did me in. I put it back on the pool but it probably is not working correctly, but I am reluctant to buy a new one and ruin it too. Maybe I will just float some tabs for the fall/winter.
- Jun 22, 2014
- 47,840
- Pool Size
- 17888
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- CircuPool RJ-45 Plus
We're about to get hit with our first cold snap of teh season, so that should lower your water temps quite a bit and reduce FC consumption. Be mindful of your CYA level and using tabs. If it's elevated, just use liquid chlorine. You should only have to add it once every 3-4 days or so.
- May 3, 2014
- 58,432
- Pool Size
- 6000
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
It would be odd to have calcium sulfate unless you are adding products that leave behind sulfate. Those are dry acid (sodium bisulfate) and non chlorine shock (monopersulfate and other variations). Calcium hypochlorite adds calcium chloride, which will show in your CH level.
Can you post a full set of water chemistry test results?
Can you post a full set of water chemistry test results?
Try this.I soaked it for hours with more acid, and there is still some calcium.


Hercules Haymaker Tankless Water Heater Descaler 35230 - The Home Depot
Hercules Haymaker Tankless Water Heater Descaler quickly and easily dissolves inorganic deposits that restrict flow to restore circulation and heat transfer efficiency. It's safe for potable water systems
www.homedepot.com
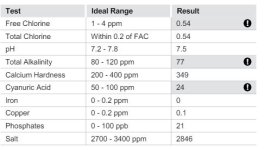
I am a pool-lazy person and I have the TFP kit but it is old and probably needs new reagents. I go to...the pool store....These are results from October 20. I know, I need to bite the bullet and learn.
I only clean the salt cell when the "check cell" light flashes, as it is very difficult for me to take off and replace by myself, and it is a pain to clean too.
I saw this on YouTube and I felt like it described our conditions in the Dallas - Fort Worth area, but I don't know if anyone else here has run into scale that won't dissolve. This particular cell has only needed cleaning once in the about five years since I have had it, so I was surprised to see that much build-up and even more surprised that it wouldn't dissolve.
Thanks for any help/ideas.
A citric acid, phosphoric acid or lactic acid formula would probably be preferable to a hydrochloric acid formula.

- May 3, 2014
- 58,432
- Pool Size
- 6000
- Surface
- Fiberglass
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-40
Do get your test kit refilled. I suspect the CH is much higher (pool stores are horrible at that test). The TA is high (they say low of course) and thus scaling tendency was high.
Can you show the scale?
It it clear or white?
Is it a shaped crystal?
Is there any color like blue, turquoise, green or black?
Calcium sulfate crystals tend to be clear with a swallowtail structure.
In the below case, they used boiling acid, but that is very dangerous.
@onBalance was involved in this case.


 www.aquamagazine.com
www.aquamagazine.com

"I called up my partner, Kim Skinner, and told him the facts of the case.
After some consideration, he suggested that the deposits might be another form of calcium than the normal calcium carbonate — such as calcium sulfate.
Calcium sulfate, CaSO4 (commonly called gypsum) can form crystals as evaporates in pools, and they are distinctive from the common calcium carbonate precipitates you often see in several key ways: First, they are softer (only 2 on the Mohs scale as opposed to calcium carbonate, which is a 3).
Secondly, they are soluble in acid only if that acid is at boiling temperatures.
And finally, they form what is called a “swallow-tail” crystalline structure.
Since the deposition process is almost entirely an evaporative one, most if not all of the crystals will be at the tile or water surface line."
It it clear or white?
Is it a shaped crystal?
Is there any color like blue, turquoise, green or black?
Calcium sulfate crystals tend to be clear with a swallowtail structure.
In the below case, they used boiling acid, but that is very dangerous.
@onBalance was involved in this case.


The Mystery of the Pointed Crystals
Seemingly overnight, this pool was covered in sharp crystals. Where did they come from?

"I called up my partner, Kim Skinner, and told him the facts of the case.
After some consideration, he suggested that the deposits might be another form of calcium than the normal calcium carbonate — such as calcium sulfate.
Calcium sulfate, CaSO4 (commonly called gypsum) can form crystals as evaporates in pools, and they are distinctive from the common calcium carbonate precipitates you often see in several key ways: First, they are softer (only 2 on the Mohs scale as opposed to calcium carbonate, which is a 3).
Secondly, they are soluble in acid only if that acid is at boiling temperatures.
And finally, they form what is called a “swallow-tail” crystalline structure.
Since the deposition process is almost entirely an evaporative one, most if not all of the crystals will be at the tile or water surface line."
Boiling acid might work, but that is dangerous and not something I would recommend unless you are very experienced handling dangerous chemicals.
HEDP or EDTA sequestrant might work.
HEDP or EDTA sequestrant might work.
I don't see scale on the tiles, just on the salt cell. I definitely won't be boiling acid.
- Mar 5, 2020
- 3,035
- Pool Size
- 66000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Astral Viron V35
I don't see scale on the tiles, just on the salt cell. I definitely won't be boiling acid.
That was just an example to show how this type of scale looks like and how difficult it is to remove. Does your scale on the cell plates look similar to this?
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

