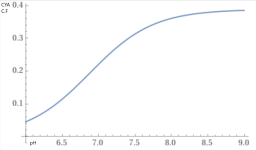
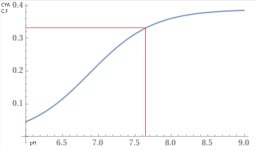
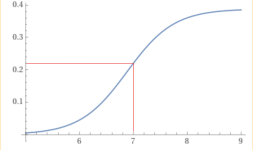
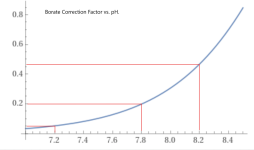
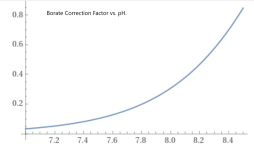
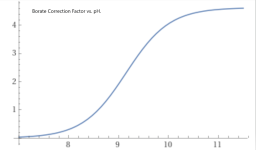
The chart at the bottom of the pH TA relationship wiki is based of a CYA of 30 ppm.
 www.troublefreepool.com
www.troublefreepool.com
How much different would this chart be with a higher CYA? in particular, 65 ppm.
PH TA Relationship - Further Reading
How much different would this chart be with a higher CYA? in particular, 65 ppm.