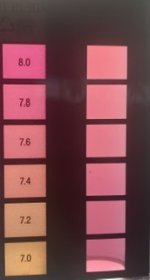
Hi all !
I'm working on a program to estimate the pH from a picture of a phenol red based comparator test to help getting more precise result than with only eyes (and of course for colorblind people).
I've based my tests using the TF-100 Comparator Block and the results so far are quite good:



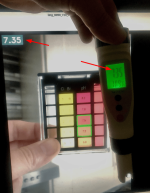
I would like to extend the detection to other pH visual test blocks and maybe add machine learning (AI) capability. For that I would need quite a lot of pictures like the one shown above with the actual pH result from a pH meter.
So, if you want to contribute, please send pictures with the real pH result in this thread
Thanks,
O.
I'm working on a program to estimate the pH from a picture of a phenol red based comparator test to help getting more precise result than with only eyes (and of course for colorblind people).
I've based my tests using the TF-100 Comparator Block and the results so far are quite good:




I would like to extend the detection to other pH visual test blocks and maybe add machine learning (AI) capability. For that I would need quite a lot of pictures like the one shown above with the actual pH result from a pH meter.
So, if you want to contribute, please send pictures with the real pH result in this thread
Thanks,
O.



 !
!