- Aug 3, 2015
- 4
- Pool Size
- 130000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Hello, TFPers. Long time, no post. Yesterday I ran into a situation and I wanted to run it by a few of you folks that understand this deep-end chemical stuff better than this amateur.
I was late opening the pool this year and had to SLAM it to deal with the algae. All was looking great recently. The water was crystal clear and looked great. Turned the heater on and brought the temperature up to wifey-warm-enough. This weekend was the final vacuum-to-waste to clear the dead algae collected on the bottom.
Friday's test results:
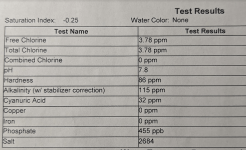
The test results above were in line with the results of my TF-100 test kit.
Following the vacuuming, I added a 22 kg bag of salt, a bottle of instant stabilizer, 4 kg of calcium (1 kg at a time mixed in a 5-gallon bucket and poured directly into the pool), and 650 ml of 31.45% muriatic acid. I then decided to add some algaecide (something I don't normally do) to ward off the little buggers. Admittedly I didn't measure and definitely poured too much in. The instructions were an initial dose of 135 ml per 10,000 litres, so 130,000 litres would need 1755 ml. As near as I can tell, I used about 2400-2666 ml or about 30%-33% of the 8L jug. No worries, I thought, it'll dissipate and be gone in a few weeks anyway. Then the SWG kicked in and now I get a white cloud out of the return jets whenever it runs. There is also a smell that is similar to chlorine but my wife described it as "soapy". The cloud disappears but the water definitely is not as clear and shiny as it was on Saturday.
The algaecide used:

My initial research was on algaecide and SWG chlorination. One article I read had a vague statement, "Algaecides can produce harmful chemical reactions if they are mixed in with high chlorine level pool water". My thought at this point is that when the cell generates the chlorine there is a localized high concentration of chlorine within the cell. Rather than going to Facebook for advice (just kidding), I asked a certain popular AI to spill the beans on whether (it thought) the statement was accurate and what the specific chemical reactions were, because that would be more helpful.
Here's the response - colour was added by me to highlight the text specific to this particular algaecide.
A test performed today with my TF-100 test kit indicates a TC reading of 3.5 and CC of 0.5, which seems to support the possibility of the formation of chloramines through this reaction.
So, in search of truth and true intelligence on such matters, I of course turn to the TFP community and its resident experts. A search of the forums turned up a few threads like this old thread from 2010 with no mention of algaecide at all, this one from 2013 and this one from a month ago. None of which though, tie the cloudy exhaust with this chemical reaction forming chloramines. All of this leads me to humbly ask you, experts, "Is this really what's going on?" Ammonium compounds are reacting with the momentarily high chlorine levels within the salt cell and produce chloramines as a result which are seen as a cloudy exhaust from the jets until it is dissipated within the expanse of the pool. And if not, what would explain the sudden rise in combined chlorine levels?
I anticipate this will resolve itself as the algaecide subsides and so it's just a matter of time but in the meantime, I'd appreciate any insight anyone might have on this subject.
Kind regards,
Hubbs
I was late opening the pool this year and had to SLAM it to deal with the algae. All was looking great recently. The water was crystal clear and looked great. Turned the heater on and brought the temperature up to wifey-warm-enough. This weekend was the final vacuum-to-waste to clear the dead algae collected on the bottom.
Friday's test results:

The test results above were in line with the results of my TF-100 test kit.
Following the vacuuming, I added a 22 kg bag of salt, a bottle of instant stabilizer, 4 kg of calcium (1 kg at a time mixed in a 5-gallon bucket and poured directly into the pool), and 650 ml of 31.45% muriatic acid. I then decided to add some algaecide (something I don't normally do) to ward off the little buggers. Admittedly I didn't measure and definitely poured too much in. The instructions were an initial dose of 135 ml per 10,000 litres, so 130,000 litres would need 1755 ml. As near as I can tell, I used about 2400-2666 ml or about 30%-33% of the 8L jug. No worries, I thought, it'll dissipate and be gone in a few weeks anyway. Then the SWG kicked in and now I get a white cloud out of the return jets whenever it runs. There is also a smell that is similar to chlorine but my wife described it as "soapy". The cloud disappears but the water definitely is not as clear and shiny as it was on Saturday.
The algaecide used:

My initial research was on algaecide and SWG chlorination. One article I read had a vague statement, "Algaecides can produce harmful chemical reactions if they are mixed in with high chlorine level pool water". My thought at this point is that when the cell generates the chlorine there is a localized high concentration of chlorine within the cell. Rather than going to Facebook for advice (just kidding), I asked a certain popular AI to spill the beans on whether (it thought) the statement was accurate and what the specific chemical reactions were, because that would be more helpful.
Here's the response - colour was added by me to highlight the text specific to this particular algaecide.
The specific chemical reactions depend on the type of algaecide and the existing chemicals in the pool, but the quote is essentially correct. Some algaecides may react with chlorine to produce potentially harmful byproducts.
Pool algaecides typically contain various compounds, such as copper-based chemicals (like copper sulfate), quaternary ammonium compounds (quats), or polymeric biguanides. The reactions of these algaecides with chlorine can be complex and not entirely predictable.
Here's a simplified explanation for some potential reactions:
- Copper-based algaecides and chlorine: Copper can react with chlorine to form copper chloride. In large concentrations, copper chloride can cause staining on pool surfaces and lead to green-colored water. Moreover, it may cause eye, skin, and respiratory irritations in people.
- Quaternary ammonium compounds and chlorine: Quats can react with chlorine to form chloramines, which are responsible for the "chlorine smell" often associated with pools. They can cause eye and skin irritation and respiratory problems.
- Polymeric biguanides and chlorine: Biguanides don't typically react directly with chlorine. However, biguanides and chlorine are incompatible as sanitizers. If mixed, they can cause water cloudiness and may leave a residue on pool surfaces.
A test performed today with my TF-100 test kit indicates a TC reading of 3.5 and CC of 0.5, which seems to support the possibility of the formation of chloramines through this reaction.
So, in search of truth and true intelligence on such matters, I of course turn to the TFP community and its resident experts. A search of the forums turned up a few threads like this old thread from 2010 with no mention of algaecide at all, this one from 2013 and this one from a month ago. None of which though, tie the cloudy exhaust with this chemical reaction forming chloramines. All of this leads me to humbly ask you, experts, "Is this really what's going on?" Ammonium compounds are reacting with the momentarily high chlorine levels within the salt cell and produce chloramines as a result which are seen as a cloudy exhaust from the jets until it is dissipated within the expanse of the pool. And if not, what would explain the sudden rise in combined chlorine levels?
I anticipate this will resolve itself as the algaecide subsides and so it's just a matter of time but in the meantime, I'd appreciate any insight anyone might have on this subject.
Kind regards,
Hubbs

