A lot of literature will say that pH should be kept at a certain range for bather comfort or for chlorine effectiveness.
TFP debunks the chlorine aspect of this through the FC/CYA ratio and I haven’t actually read it but I’m pretty sure the bather comfort side of this is not true as well.
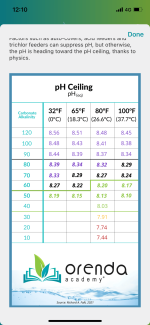
So, what is the issue if pH exceeds 8? If I’ve got my levels set up that the pH ceiling is say, 8.1, is there a problem with letting it sit at this and not using MA at all?
Edit: making sure the LSI is in check
TFP debunks the chlorine aspect of this through the FC/CYA ratio and I haven’t actually read it but I’m pretty sure the bather comfort side of this is not true as well.
So, what is the issue if pH exceeds 8? If I’ve got my levels set up that the pH ceiling is say, 8.1, is there a problem with letting it sit at this and not using MA at all?
Edit: making sure the LSI is in check
Last edited:





.png)