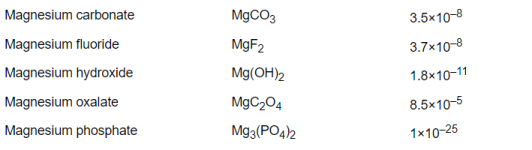Is pH 8.7 acceptable if csi is zero?
- Thread starter key1cc
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
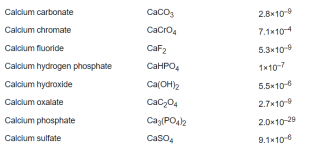
Percentage bicarbonate.
Below a pH of 8.3, the bicarbonate is becoming carbon dioxide.
Above a pH of 8.3, the bicarbonate is becoming carbonate.
CO2 + H2O <-> H2CO3 <-> H+ + HCO3-<-> 2H+ + CO32-
 www.wolframalpha.com
www.wolframalpha.com
Below a pH of 8.3, the bicarbonate is becoming carbon dioxide.
Above a pH of 8.3, the bicarbonate is becoming carbonate.
CO2 + H2O <-> H2CO3 <-> H+ + HCO3-<-> 2H+ + CO32-

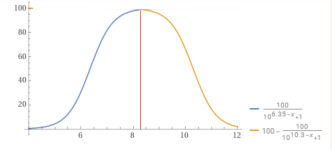
plot y = 100÷(1+10^(6.35 – x)) and y = 100-(100÷(1+10^(10.3 – x))), x from 4 to 12 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
Percentage bicarbonate.
Below a pH of 8.3, the bicarbonate is becoming carbon dioxide.
Above a pH of 8.3, the bicarbonate is becoming carbonate.
CO2 + H2O <-> H2CO3 <-> H+ + HCO3-<-> 2H+ + CO32-
View attachment 547405
plot y = 100÷(1+10^(6.35 – x)) and y = 100-(100÷(1+10^(10.3 – x))), x from 4 to 12 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.www.wolframalpha.com
Soooo....Between pH 7.5 and pH 9.0, the bulk of the ions are present as bicarbonate however significantly more carbonate forms at higher pH and significantly less (due to carbon dioxide formation) at lower pH, so in typical swimming pool ranges, the carbon dioxide, bicarbonate, and carbonate are always in a pH dependent equilibrium.
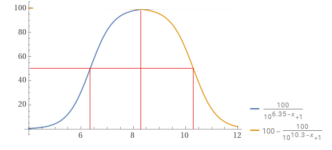
The pKa determines the percentage vs. the pH.
When the pH = pKa, then that is where the molar percentage is 50% for each.
For carbon dioxide, the pKa is about 6.35.
For bicarbonate, the pKa is about 10.3.
So, the percentage bicarbonate is 50% or higher for the pH range of about 6.35 to 10.3 and 8.3 is about in the center where the percentage is close to 100% but slightly less than 100%.
_____________________________________________________________________________________
Percentage bicarbonate.
You can use the formulas to calculate the percentage of bicarbonate vs. carbon dioxide or vs. carbonate.
100÷(1+10^(6.35 – 8.3)) = 98.8904% bicarbonate vs. carbon dioxide.
100-(100÷(1+10^(10.3 – 8.3))) = 99.0099% bicarbonate vs. carbonate.
So, at a pH of 8.3, you have have a mix of carbon dioxide, bicarbonate and carbonate.
You would have about 1% CO2, 98% bicarbonate and 1% carbonate.
You would have about 1.09873% CO2, 97.922% bicarbonate and 0.979221% carbonate.
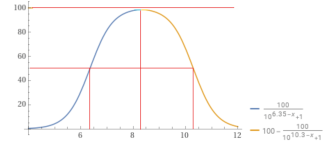
___________________________________________________________________________________
Bicarbonate vs. carbonate.
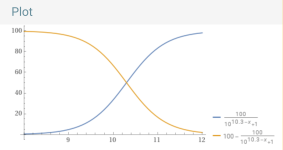
____________________________________________________________________________
Carbon Dioxide vs. Bicarbonate.
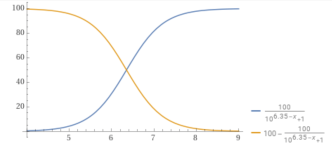
 www.wolframalpha.com
www.wolframalpha.com
When the pH = pKa, then that is where the molar percentage is 50% for each.
For carbon dioxide, the pKa is about 6.35.
For bicarbonate, the pKa is about 10.3.
So, the percentage bicarbonate is 50% or higher for the pH range of about 6.35 to 10.3 and 8.3 is about in the center where the percentage is close to 100% but slightly less than 100%.
_____________________________________________________________________________________
Percentage bicarbonate.

You can use the formulas to calculate the percentage of bicarbonate vs. carbon dioxide or vs. carbonate.
100÷(1+10^(6.35 – 8.3)) = 98.8904% bicarbonate vs. carbon dioxide.
100-(100÷(1+10^(10.3 – 8.3))) = 99.0099% bicarbonate vs. carbonate.
So, at a pH of 8.3, you have have a mix of carbon dioxide, bicarbonate and carbonate.
You would have about 1% CO2, 98% bicarbonate and 1% carbonate.
You would have about 1.09873% CO2, 97.922% bicarbonate and 0.979221% carbonate.

___________________________________________________________________________________
Bicarbonate vs. carbonate.

____________________________________________________________________________
Carbon Dioxide vs. Bicarbonate.

plot y = 100÷(1+10^(6.35 – x)) and y = 100-(100÷(1+10^(6.35 – x))), x from 4 to 9 - Wolfram|Alpha
Wolfram|Alpha brings expert-level knowledge and capabilities to the broadest possible range of people—spanning all professions and education levels.
Last edited:
To answer the original question: Yes, the CSI does and will accurately provide guidance on whether water will be scaling or in an etching condition when the pH goes very high or low in a body of water. However, as with many situations, other factors need to be considered that might affect the end result.
I have experience with maintaining many pools with pH levels of 8.0 to 8.3 without any scaling.
I also have experimented with some pools keeping the pH in the range of 9.0 to 10.0 and keeping the CSI within the balance range. No scale. But that was in vinyl pools, not plaster pools. Scale did develop in plaster pools BECAUSE the plaster is not impervious to the water. And another way to describe this is to say that plaster is slightly permeable and can bleed out calcium hydroxide (with certain conditions) and that can significantly affect the pool water at the immediate plaster surface. I believe that James mentioned this aspect earlier. I believe that factor is at play here.
In my opinion, this plaster pool (in this thread) is likely to be somewhat new, and new plaster will likely emit plaster compounds into the water for a period of time and raise the pH. I suspect this is the case with this pool because the pH seems to want to go very high and will likely cause scale to develop because the CSI will be much higher at the plaster surface than in the rest of the pool water. That is not a good sign for the plaster finish, and is not good for the pool water.
With new plaster pools under a year old and during the winter, I would strongly suggest to keep the pH below 8.0 and lower it immediately whenever it goes above 8.0. Otherwise, scale is likely to develop due to the new plaster effects.
I have experience with maintaining many pools with pH levels of 8.0 to 8.3 without any scaling.
I also have experimented with some pools keeping the pH in the range of 9.0 to 10.0 and keeping the CSI within the balance range. No scale. But that was in vinyl pools, not plaster pools. Scale did develop in plaster pools BECAUSE the plaster is not impervious to the water. And another way to describe this is to say that plaster is slightly permeable and can bleed out calcium hydroxide (with certain conditions) and that can significantly affect the pool water at the immediate plaster surface. I believe that James mentioned this aspect earlier. I believe that factor is at play here.
In my opinion, this plaster pool (in this thread) is likely to be somewhat new, and new plaster will likely emit plaster compounds into the water for a period of time and raise the pH. I suspect this is the case with this pool because the pH seems to want to go very high and will likely cause scale to develop because the CSI will be much higher at the plaster surface than in the rest of the pool water. That is not a good sign for the plaster finish, and is not good for the pool water.
With new plaster pools under a year old and during the winter, I would strongly suggest to keep the pH below 8.0 and lower it immediately whenever it goes above 8.0. Otherwise, scale is likely to develop due to the new plaster effects.
Last edited:
This can be mitigated with frequent brushing and good continuous circulation.plaster is slightly permeable and can bleed out calcium hydroxide (with certain conditions) and that can significantly affect the pool water at the immediate plaster surface.
The effect is mostly due to water that does not move and you get a layer of high pH water directly adjacent to the plaster.
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
So you are saying that I should ignore the advice that some have given to just winterize my pool and forget about it until spring? Because if I follow that advice, that means no pH adjusting, no brushing, and no circulation creating a stagnant environment with very high pH not only close to the surface but throughout this entire new plaster pool resulting in scale.This can be mitigated with frequent brushing and good continuous circulation.
The effect is mostly due to water that does not move and you get a layer of high pH water directly adjacent to the plaster.
On the other hand I can go out to my pool every few days as i have been and add some acid and circulate a couple hours….throughout the entire winter no matter how brutal the weather…….as long as the pool isn’t frozen.
Well……Neither of them are good options long term.
key1cc, it is an unfortunate situation you have with your pool.
I do have a question that I am curious about. Did you your calcium hardness go actually go from 250 ppm to 625 ppm?
If so, what time span did that occur? When was your pool plastered?
Can you test your tap or fill water for the pH, TA, and CH?
I do have a question that I am curious about. Did you your calcium hardness go actually go from 250 ppm to 625 ppm?
If so, what time span did that occur? When was your pool plastered?
Can you test your tap or fill water for the pH, TA, and CH?
In my opinion, it is never a good idea to let a plaster pool sit for long periods of time without being circulated and balanced.
This is especially true if the plaster is less than 1 year old.
This is especially true if the plaster is less than 1 year old.
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
My pool was plastered 5-3-23 ...then filled.key1cc, it is an unfortunate situation you have with your pool.
I do have a question that I am curious about. Did you your calcium hardness go actually go from 250 ppm to 625 ppm?
If so, what time span did that occur? When was your pool plastered?
Can you test your tap or fill water for the pH, TA, and CH?
My Calcium results were as follows: 200 on 5/11 when 1st tested, 225 on 5/13, 325 on 6/13, 375 on 7/10, heavy rains and draining some water then refilling = 325 on 7/17, 400 on 8/7, 450 on 9/4, 500 on 10/20. For winterization purposes I then added 8lbs of 85% CaCl bringing it up to 550 on 10/22, It was 575 on 11/19. Raining and draining up to 12/11 took it back down to 525. I added a 10lb bag of 100% CaCl which bought me to 625 three days later. Record rainfall in mid December (my rain guage measured over 8 inches in a 2 day period) followed by draining and refilling brought Ca down to 500ppm where I added another 10lb bag CaCl bringing it back up to 600ppm. More raining and draining puts me at 550 ppm where I am today.
Tap water was used to fill pool: Tap water Alkalinity is 70ppm and Ca = 125ppm and pH=7.18
Also, I did not start paying attention to CSI until 9/25....before that I used my TF-Pro kit to stay in the ranges
Last edited:
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
Also, I attributed the constant rising calcium to leeching from the plaster. So, I have been trying to keep CSI in the positive side in recent weeks. For me that is pH 8.1 to 8.4 pH........ = CSI 0.0 to 0.3
Latest Readings: Alkalinity=55, calcium hardness=550, cya=60, water temp = 42, salt=2600
Latest Readings: Alkalinity=55, calcium hardness=550, cya=60, water temp = 42, salt=2600
If you were adding CaCl to raise the calcium level during the summer and fall, why do you suggest that there was calcium leeching going on? Do you have an idea of how much of the calcium increase was from leeching versus CaCl additions? I agree with you that with the pH going to 8.7 indicates leeching.
I just re-read post#50 and it appears that the calcium hardness increased from 200 to 500 ppm without CaCl additions, and then you raise it higher in the fall with CaCl additons. Is that correct?
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
I only added calcium on the dates I indicated above. On 10-22 was my 1st calcium addition hoping that if I further saturate the water with calcium it would stop leeching it. Prior to me adding any calcium the Calcium rose on its own from 200 to 500 ppm.....that I suspect was leechingIf you were adding CaCl to raise the calcium level during the summer and fall, why do you suggest that there was calcium leeching going on? Do you have an idea of how much of the calcium increase was from leeching versus CaCl additions? I agree with you that with the pH going to 8.7 indicates leeching.
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
CorrectI just re-read post#50 and it appears that the calcium hardness increased from 200 to 500 ppm without CaCl additions, and then you raise it higher in the fall with CaCl additons. Is that correct?
Okay, got it. I should have figured that out the first time I read that post.
So it appears that the calcium level increased almost three-fold during the summer when the water temp was higher and with balanced water that you were trying to maintain. Would you agree that for the most part (during the summer) that the CSI was fairly balanced even though you were keeping track of that Index at the time?
If so, I hate to be the bearer of bad news, but it would likely that the plastering quality may have lacked somewhat. This would mean that you do need to keep a slight positive CSI and also brush the plaster at much as possible during the winter. Hopefully, it won't freeze over and prevent you from doing that. In the future, when the plaster is more cured, you may not have to perform the brushing as much as you need to do this winter.
You should read this post of mine.
 www.troublefreepool.com
www.troublefreepool.com
So it appears that the calcium level increased almost three-fold during the summer when the water temp was higher and with balanced water that you were trying to maintain. Would you agree that for the most part (during the summer) that the CSI was fairly balanced even though you were keeping track of that Index at the time?
If so, I hate to be the bearer of bad news, but it would likely that the plastering quality may have lacked somewhat. This would mean that you do need to keep a slight positive CSI and also brush the plaster at much as possible during the winter. Hopefully, it won't freeze over and prevent you from doing that. In the future, when the plaster is more cured, you may not have to perform the brushing as much as you need to do this winter.
You should read this post of mine.
Why Are Calcium Crystals Forming in Pools?
In recent years, calcium crystals have been forming during the winter at a higher rate in some newly plastered swimming pools. From the reports we have heard, this problem seems to occur more often in the northeast of the country. Apparently, pool builders, plasterers, and service techs involved...
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
Thanks for sharing your link and the subsequent Orenda link.Okay, got it. I should have figured that out the first time I read that post.
So it appears that the calcium level increased almost three-fold during the summer when the water temp was higher and with balanced water that you were trying to maintain. Would you agree that for the most part (during the summer) that the CSI was fairly balanced even though you were keeping track of that Index at the time?
If so, I hate to be the bearer of bad news, but it would likely that the plastering quality may have lacked somewhat. This would mean that you do need to keep a slight positive CSI and also brush the plaster at much as possible during the winter. Hopefully, it won't freeze over and prevent you from doing that. In the future, when the plaster is more cured, you may not have to perform the brushing as much as you need to do this winter.
You should read this post of mine.
Why Are Calcium Crystals Forming in Pools?
In recent years, calcium crystals have been forming during the winter at a higher rate in some newly plastered swimming pools. From the reports we have heard, this problem seems to occur more often in the northeast of the country. Apparently, pool builders, plasterers, and service techs involved...www.troublefreepool.com
I'm seeing lots of very similar thinking yet different opinions (if that's possible). Also, it's good to know that this is not something new that is only impacting me. In fact, it sounds like the issue is quite common in the Northeast with new plaster pools.... and by issue, I mean constant rising ph. Fortunately for me, I haven't had any issues other than constantly rising ph..... no scale, no crystals, no dust, nothing.
If my plaster is of poor quality, then it is not visually evident to me. I have not brushed my pool since closing other than in the open corners to help mix in acid.
The best thing that can happen for me at this point is one of the inventors out there to invent a stand-alone "low power usage" water circulator that I could just drop in the center of the pool for the winter..... in the meantime, the weather has been reasonably agreeable with next week forecasted as having a couple days in the 50's. My water is in the low 40's so fingers crossed I make it to "plaster cure completion" before pool freezes if it freezes at all this season.
- Oct 25, 2022
- 56
- Pool Size
- 14000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-9)
Yes it was mostly balanced except maybe for the 3 or 4 days I dropped the pH to ~7 in an attempt to lower the alkalinity.Would you agree that for the most part (during the summer) that the CSI was fairly balanced even though you were keeping track of that Index at the time?
- Jul 21, 2013
- 54,613
- Pool Size
- 35000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Looks like this winter in NJ you will luck out with warm weather for most of the winter which will let you tend to your pool most of the time.
If we do gets a few weeks of freezing it should not cause you much of a problem.
If we do gets a few weeks of freezing it should not cause you much of a problem.
I need to proof read my comments before clicking the reply button. I know that you "weren't" keeping track of the CSI during the summer, but are confirming now that your pool water was very likely balanced based on the levels you were maintaining most of the time during the summer.
You probably won't see any visible problems with your plaster. Hopefully, it will look good for many years.
The fact that you are keeping the pH under control and the CSI within the balanced range is probably helping a lot to prevent calcium crystals or typical scale forming on the plaster.
It is common that the pH will want to rise naturally to 8.0 - 8.3 when the (carbonate) alkalinity is anywhere between 60 ppm and 120 ppm. If the pH goes higher than that means other issues are at play.
I hope you make it through the winter without major problems. It is obvious that you have a good understanding of pool water chemistry. Hope all goes well from here on.
You probably won't see any visible problems with your plaster. Hopefully, it will look good for many years.
The fact that you are keeping the pH under control and the CSI within the balanced range is probably helping a lot to prevent calcium crystals or typical scale forming on the plaster.
It is common that the pH will want to rise naturally to 8.0 - 8.3 when the (carbonate) alkalinity is anywhere between 60 ppm and 120 ppm. If the pH goes higher than that means other issues are at play.
I hope you make it through the winter without major problems. It is obvious that you have a good understanding of pool water chemistry. Hope all goes well from here on.