Whenever you are measuring something, like temperature, salinity, pressure etc. you have to think in terms of probability, accuracy, resolution and tolerance.
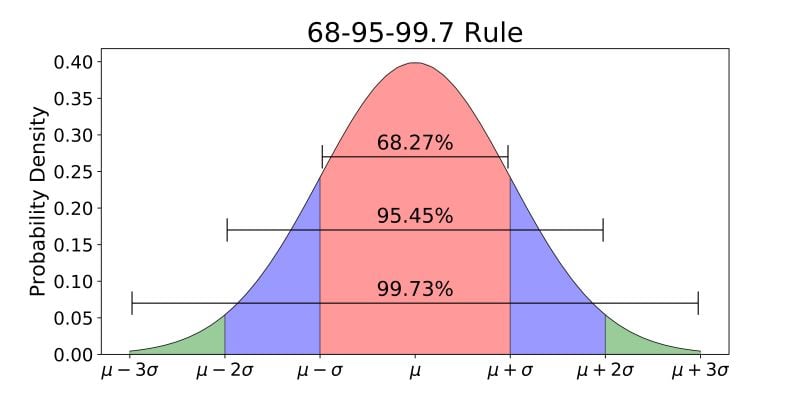
If you get a reading, there is always a probability distribution that provides a range of results.
For example, a reading of 3,200 ppm from a meter might be +/- 100 ppm to within 1 standard deviation, which means that there is a 68.27% chance that the actual number is between 3,100 ppm and 3,300 ppm.
If you want 95.45% certainty. that might be +/- 200ppm.
99.73% certainty might be +/-300 ppm.
Even then, there is always the possibility of some sort of defect or malfunction that makes the test inaccurate.
Maybe the batteries are going bad or the meter needs to be cleaned or calibrated or the meter is malfunctioning or the temperature sensor is off etc.
And, there is always the possibility that there is some sort of defect in the process of doing the test or in getting a sample.
Maybe the sample bottle is contaminated.
Maybe the person uses the wrong sample size or the wrong multiplier.
Even if the sample size is off by a tiny bit, it can make the result inaccurate.
Maybe the person is not holding the dropper correctly etc.
Maybe the salt has been added but it is sitting on the floor and it is not mixed into the water.
Maybe there is rain water on top and the cell is getting the wrong reading.