Funny you mentioned that as I was just thinking about doing that to save reagent. In any case, it looks like I'll be placing an order to TFTestkits sooner than laterYou can try diluting a water sample or using a 5mL sample so that the conversion is 1ppm/drop. You’re going to burn through a lot of reagent with numbers that high.
First-time SLAM with unexpected CC and pH
- Thread starter mbender71
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Quick update! You won't believe this @JoyfulNoise, but it's been a little over 30 minutes since I added the muriatic acid, and the pool is AMAZINGLY more clear. I can even see the remaining light staining on the plaster. I'm not a chemist by any means, but perhaps the increase in pH by all the chlorine precipitated something in the pool water, and when the pH was brought back down to the high 7's (I haven't tested yet), something dissolved back into the solution? I'll report my beginning OCLT tests after I perform them at sunset. High hopes here, but we'll see!
- May 23, 2015
- 24,440
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Water clarity can change with pH , it’s not unusual. And if the pH was really high, you can get precipitates to form. Best to keep the pH below 8.0 so you don’t cause any unwanted scale.
Understood, and thank you again! For giggles, I performed a quick pH test with an off-brand pool test kit (chemical) that requires a drop of de-chlorinator. The result was 7.8. I haven't performed the electronic pH test yet, BUT doesn't it say something about the kind of chlorine in the pool if the chemical pH test kit is not affected? I've often read that testing pH with very high chlorine levels is inaccurate. I'm sorry if this is a silly question. Perhaps it's an incorrect question since I have too many chemical parameters floating in my headWater clarity can change with pH , it’s not unusual. And if the pH was really high, you can get precipitates to form. Best to keep the pH below 8.0 so you don’t cause any unwanted scale.
- May 23, 2015
- 24,440
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Understood, and thank you again! For giggles, I performed a quick pH test with an off-brand pool test kit (chemical) that requires a drop of de-chlorinator. The result was 7.8. I haven't performed the electronic pH test yet, BUT doesn't it say something about the kind of chlorine in the pool if the chemical pH test kit is not affected? I've often read that testing pH with very high chlorine levels is inaccurate. I'm sorry if this is a silly question. Perhaps it's an incorrect question since I have too many chemical parameters floating in my head
Chlorosulfamate is not a strong enough oxidizer to chlorinate phenol red into chlorphenol red. Since most of the sanitizer in your pool is currently in the form of chlorosulfamate, it’s effect on the pH indicator is minimal.
The first OCLT *evening* test results are in :
FC: 8
CC: 48
TC: 56
pH: 7.65
Water Temp: 76F
It's been 5 hours since the last set of tests, and FC dropped 3.5 ppm (unreliable), and CC dropped 12 ppm. The pH adjusted a little below the targetted 7.8 as suggested by @JoyfulNoise. I'll retest the water before sunrise tomorrow to check how the chlorine levels are performing for the first full OCLT. I wonder if the pH will drift towards 7.8 overnight.
FC: 8
CC: 48
TC: 56
pH: 7.65
Water Temp: 76F
It's been 5 hours since the last set of tests, and FC dropped 3.5 ppm (unreliable), and CC dropped 12 ppm. The pH adjusted a little below the targetted 7.8 as suggested by @JoyfulNoise. I'll retest the water before sunrise tomorrow to check how the chlorine levels are performing for the first full OCLT. I wonder if the pH will drift towards 7.8 overnight.
The first OCLT *morning* test results are in :
FC: 8
CC: 46
TC: 54
pH: 7.70
Water Temp: 74F
The overnight results are interesting. In my amateur interpretation, the pool is pretty darn free of anything living as the FC (unreliable), and CC hardly dropped at all. The real test will be this evening when I test the water after a day's worth of sun. I hope that the FC & CC drop a fair amount (like yesterday) which (I think) would conclude that it is only a matter of "sunny days" before chlorine levels drop to "normal" levels. I already see the pH creeping up slowly, so I will keep an eye on that as well.
The pool will be above the required FC/TC of 20 ppm for another couple of days (I'm pretty sure) on its own due to my over-chlorination. The water is crystal clear, and the OCLT shows hardly any movement of FC/TC. The "bigger" question is, "What is the acceptable amount of Chlorosulfamate in a pool before safely going in?" If it's a cruddy oxidizer, then perhaps it's more of an annoyance than harmful.
FC: 8
CC: 46
TC: 54
pH: 7.70
Water Temp: 74F
The overnight results are interesting. In my amateur interpretation, the pool is pretty darn free of anything living as the FC (unreliable), and CC hardly dropped at all. The real test will be this evening when I test the water after a day's worth of sun. I hope that the FC & CC drop a fair amount (like yesterday) which (I think) would conclude that it is only a matter of "sunny days" before chlorine levels drop to "normal" levels. I already see the pH creeping up slowly, so I will keep an eye on that as well.
The pool will be above the required FC/TC of 20 ppm for another couple of days (I'm pretty sure) on its own due to my over-chlorination. The water is crystal clear, and the OCLT shows hardly any movement of FC/TC. The "bigger" question is, "What is the acceptable amount of Chlorosulfamate in a pool before safely going in?" If it's a cruddy oxidizer, then perhaps it's more of an annoyance than harmful.
- May 23, 2015
- 24,440
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
My suggestion would be to add a dose of chlorine each day that brings the FC up to shock levels (20ppm FC) each day and not worry too much about the CC reading because it is always going to be elevated with sulfamate in the water. You need to oxidize the sulfamate to get rid of it. Eventually it’s going to start to breakdown and when that happens you’ll see the CCs start to drop and then the pH and TA will begin to fall as acidic reactions occur (ammonium reacts with chlorine to form nitrogen and nitrates and in the process produces excess hydrogen ions).
Do you have any records of how much of the sulfamic acid you added?
Do you have any records of how much of the sulfamic acid you added?
My suggestion would be to add a dose of chlorine each day that brings the FC up to shock levels (20ppm FC) each day and not worry too much about the CC reading because it is always going to be elevated with sulfamate in the water. You need to oxidize the sulfamate to get rid of it. Eventually it’s going to start to breakdown and when that happens you’ll see the CCs start to drop and then the pH and TA will begin to fall as acidic reactions occur (ammonium reacts with chlorine to form nitrogen and nitrates and in the process produces excess hydrogen ions).
Do you have any records of how much of the sulfamic acid you added?
I'm a little confused. Right now, FC can not be accurately measured, I thought, and therefore we are using TC (FC+CC) to dose the chlorine. I believe I got myself into this situation (too much chlorine) due to the inability to measure FC. After the OCLT results, I think I can safely say that any of the chlorine in the pool is not reacting with anything, and therefore the pool is "inert" in regards to living organisms. Only the residual chemicals are left to remove. If sulfamates are not that harmful (i.e. I'm not a fish breathing it), then couldn't I oxidize the residual sulfamate naturally over time by the SWG and not continue this dumping of large amounts of chlorine (which got me into this situation in the first place)? It seems like an endless cycle if I do since the pH will rise, the pool will go cloudy, then I have to add muriatic acid again to bring the pH to 8.0, and around we go.
As for how much sulfamic acid was added, that is a little harder to tell. The treatment was 10lbs of their copper treatment (in granular form), but the labeling does not provide any clues to concentration. In addition to the copper treatment, I am also instructed to add Jack's Magic Purple Stuff (which is phosphoric acid and the label gives no hint at concentration), at a rate of 12oz every week. I'm scheduled to add that tomorrow to keep up with the program. I'm not a chemist, but this might add to the already complicated equation.
I apologize if I'm missing something here :-(
- May 23, 2015
- 24,440
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Your FC measurement isn't "inaccurate" at all ... it's 8ppm. Your chlorosulfamate levels are approximately 46ppm. That is a chemical fact.
You need to get rid of the sulfamate. The only way to do that is for chlorine to slowly oxidize it over time and by breaking the sulfur-nitrogen bond thus creating a bisulfate anion (which eventually turns into sulfate) and an ammonium anion. The ammonium will then be further oxidized into nitrogen/nitrate and, in the process, release hydrogen ions (H+). Those reactions are acidic and will drop the pH over time.
The less chlorine you add, the slower the process will be. This is why I suggested adding at least a dose of liquid chlorine that gets you up to SLAM levels of FC and then let it react with the sulfamate however it wants. Your SWG cannot create chlorine fast enough and, honestly speaking, there's no way to know what potentially damaging effect chlorosulfamate reactions can have on the catalyst coating. It might be none or it could be something. If it were my pool, I would switch off the SWG to protect it and then work on getting rid of the sulfamate using liquid chlorine exclusively. The CC's generated from the chlorosulfamate are not harmful. They are a weak disinfectant (slow to kill pathogens) and they do no oxidizing so you need some measurable levels of FC in the water to ensure that it is sanitary.
The pH rise from liquid chlorine is minimal. In fact, it's mostly pH neutral since the pH rise that occurs from adding chlorine is offset eventually by the acidic reactions that chlorine has with most stuff in the water. Since you have access to a pH probe, you can easily keep an eye on your pH and adjust as necessary with muriatic acid.
Jack's Purple Stuff is HEDP, not phosphoric acid. There's no issue with adding that, it has minimal effects on pH or chlorine levels. You can choose to add it or not, that's up to you.
You need to get rid of the sulfamate. The only way to do that is for chlorine to slowly oxidize it over time and by breaking the sulfur-nitrogen bond thus creating a bisulfate anion (which eventually turns into sulfate) and an ammonium anion. The ammonium will then be further oxidized into nitrogen/nitrate and, in the process, release hydrogen ions (H+). Those reactions are acidic and will drop the pH over time.
The less chlorine you add, the slower the process will be. This is why I suggested adding at least a dose of liquid chlorine that gets you up to SLAM levels of FC and then let it react with the sulfamate however it wants. Your SWG cannot create chlorine fast enough and, honestly speaking, there's no way to know what potentially damaging effect chlorosulfamate reactions can have on the catalyst coating. It might be none or it could be something. If it were my pool, I would switch off the SWG to protect it and then work on getting rid of the sulfamate using liquid chlorine exclusively. The CC's generated from the chlorosulfamate are not harmful. They are a weak disinfectant (slow to kill pathogens) and they do no oxidizing so you need some measurable levels of FC in the water to ensure that it is sanitary.
The pH rise from liquid chlorine is minimal. In fact, it's mostly pH neutral since the pH rise that occurs from adding chlorine is offset eventually by the acidic reactions that chlorine has with most stuff in the water. Since you have access to a pH probe, you can easily keep an eye on your pH and adjust as necessary with muriatic acid.
Jack's Purple Stuff is HEDP, not phosphoric acid. There's no issue with adding that, it has minimal effects on pH or chlorine levels. You can choose to add it or not, that's up to you.
Last edited:
- May 23, 2015
- 24,440
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
The Jacks #2 chemical is pretty much pure granular sulfamic acid. At 10lbs in 11,000 gallons of water you roughly added around 110ppm of sulfamate to the pool. It’s going to take a long time to clear that all out.
The sulfamic acid is probably holding onto the copper from the stains keeping it sequestered.
As the sulfamic breaks down, the copper will be released and it can cause new stains.
Dilution would get rid of the sulfamic and copper and reduce the CC and copper faster.
Do not drain unless you are sure that the pool will not float.
As the sulfamic breaks down, the copper will be released and it can cause new stains.
Dilution would get rid of the sulfamic and copper and reduce the CC and copper faster.
Do not drain unless you are sure that the pool will not float.
In my opinion, there is no reason to SLAM if you are passing am OCLT.If sulfamates are not that harmful (i.e. I'm not a fish breathing it), then couldn't I oxidize the residual sulfamate naturally over time by the SWG and not continue this dumping of large amounts of chlorine (which got me into this situation in the first place)?
Just maintain the TC at about 15% of the CYA and verify that it is enough by doing an OCLT.
In any case, I would recommend significant dilution.
You can do a continuous dilution by adding water and draining at the exact same rate.
This is not as efficient as draining and refilling, but it can be a lot safer and easier.
In my opinion, there is no reason to SLAM if you are passing am OCLT.
Just maintain the TC at about 15% of the CYA and verify that it is enough by doing an OCLT.
In any case, I would recommend significant dilution.
You can do a continuous dilution by adding water and draining at the exact same rate.
This is not as efficient as draining and refilling, but it can be a lot safer and easier.
Hi there @JamesW, and thank you so much for chiming in! It's funny since I was *JUST* reading over a thread right now that you and @JoyfulNoise participated in with a member who also used Jack's Magic Copper treatment (though for scale, not copper). It does appear that I've stepped into some questionable territory with my use of Jack's Magic Copper Treatment. I will say that my two-week treatment worked quite well in removing quite a bit of copper staining, so I'm happy I did it, but I don't want to do it again! I have to say that I favor the side of NOT continuing with the SLAM as my OCLT provided me with the confidence that the pool is free of living organisms (plus the water is crystal clear). I do wonder why I can't get my water a nice shade of blue (it's more turquoise), but I'm guessing that is a result of 16-year-old plaster that isn't as white as it used to be. I will hold back on chemical changes and procedures until my next water test tonight. I want to see how my FC & CC change during the stress of the sun since I know overnight it doesn't change much at all. The rate of change today will determine how I proceed, and I will report back tonight with the measurements. I will consider continuous dilution in the future as soon as I figure out a nice way of doing it (where to drain, a small pump, and an easy water supply refill water).
I am very grateful to have the deep chemistry knowledge from @JoyfulNoise and @JamesW!!! Thank you so much!
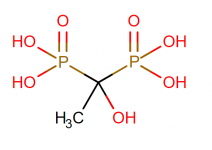
HEDP is Hydroxyethylidene Diphosphonic Acid.I am also instructed to add Jack's Magic Purple Stuff (which is phosphoric acid and the label gives no hint at concentration)
HEDP is also known as Etidronic acid, which is a type of phosphonic acid (aka Phosphorous acid).
Molecular Formula: C2H8O7P2
It will hold onto the copper for a while, but it will eventually break down into orthophosphate and it will release the copper.
So, if you don’t want the copper stains to return, you will need to keep adding the HEDP or get rid of the copper.

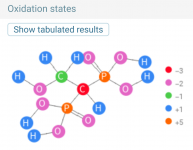
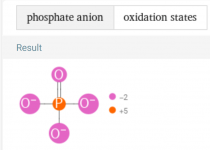
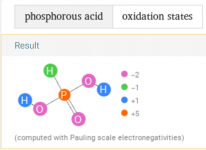
HEDP is also known as Etidronic acid, which is a type of phosphonic acid (aka Phosphorous acid).
Molecular Formula: C2H8O7P2
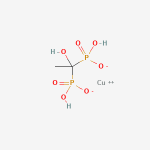
Copper etidronic acid complex.
C2H6CuO7P2
Copper(II) (1-hydroxyethylidene)bisphosphonate
The breakdown of HEDP is probably hydrolysis of the carbon-phosphorous bond and the oxidation of the carbon to carbon dioxide.
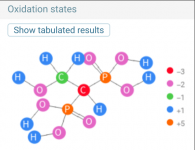
Etidronic Acid, a crystalline diphosphonate with both detergent and chelating utilities, is a tetraprotic acid with the acidity of the first proton being rather strong, to the last being rather alkaline (pKa’s: pKa1= 1.35; pKa2= 2.87; pKa3 = 7.03; and pKa4 = 11.3).
pKa1 = 1.7
pKa2 = 2.47
pKa3 = 7.28
pKa4 = 10.29
Four pKa values: 1.6, 2.7, 6.9, 11
As a consequence of ionization, HEDP is a strong complexing agent.
Molecular Formula: C2H8O7P2
Copper etidronic acid complex.
C2H6CuO7P2
Copper(II) (1-hydroxyethylidene)bisphosphonate
The breakdown of HEDP is probably hydrolysis of the carbon-phosphorous bond and the oxidation of the carbon to carbon dioxide.




Etidronic Acid, a crystalline diphosphonate with both detergent and chelating utilities, is a tetraprotic acid with the acidity of the first proton being rather strong, to the last being rather alkaline (pKa’s: pKa1= 1.35; pKa2= 2.87; pKa3 = 7.03; and pKa4 = 11.3).
pKa1 = 1.7
pKa2 = 2.47
pKa3 = 7.28
pKa4 = 10.29
Four pKa values: 1.6, 2.7, 6.9, 11
As a consequence of ionization, HEDP is a strong complexing agent.
Last edited:
- Jun 1, 2018
- 13,770
- Pool Size
- 26000
- Surface
- Vinyl
- Chlorine
- Salt Water Generator
- SWG Type
- Hayward Aqua Rite (T-15)
Mbender,
The copper came from somewhere, likely the previous service/owner using things with “blue” in the name or copper laden algaecides. Unless you want to keep adding expensive sequestrants (which have their drawbacks) to keep the copper from staining things or turning the water, hair, fingernails green you should exchange the copper laden water for fresh water. This would also take care of the sulfamic acid situation.
Since hopefully you won’t be using any TFP non recommended copper laden products from here on out the problem will be resolved & you will know where you stand with your pool.
The no drain water exchange is the safest way to do this in saturated florida.
Liquid chlorine isn’t cheap & it will take alot of it & alot of time to chew through your sulfamic acid.
 www.google.com
www.google.com
The copper came from somewhere, likely the previous service/owner using things with “blue” in the name or copper laden algaecides. Unless you want to keep adding expensive sequestrants (which have their drawbacks) to keep the copper from staining things or turning the water, hair, fingernails green you should exchange the copper laden water for fresh water. This would also take care of the sulfamic acid situation.
Since hopefully you won’t be using any TFP non recommended copper laden products from here on out the problem will be resolved & you will know where you stand with your pool.
The no drain water exchange is the safest way to do this in saturated florida.
Liquid chlorine isn’t cheap & it will take alot of it & alot of time to chew through your sulfamic acid.
Copper in Pool Water - Further Reading
Hello everyone! I performed this evening's OCLT test for comparison tomorrow morning, BUT I also wanted to see how much FC + CC would drop during the day since last night the levels barely changed. Today was a beautiful sunny day without a single cloud in the sky the entire day (I know this from looking at my solar panel production). Here are the results :
FC: 4
CC: 35
TC: 39
Water Temp: 77F
I had a 4 ppm drop in FC and an 11 ppm drop in CC today. Wow, that's a beautiful drop. So much so that I have to ask the experts (@JoyfulNoise & @JamesW) if my CC has a lesser amount of chlorosulfamate than expected. I was preparing for a very slow drop in CC due to assumed chlorosulfamate content, but at this rate, it will take just days to get my FC + CC in check. The pool is crystal clear and shimmering in the sunlight now, which makes me SUPER happy. I will perform the OCLT test in the morning before sunrise to provide a final confirmation that my SLAM is coming to an end. Thanks again to everyone who has chimed in!
FC: 4
CC: 35
TC: 39
Water Temp: 77F
I had a 4 ppm drop in FC and an 11 ppm drop in CC today. Wow, that's a beautiful drop. So much so that I have to ask the experts (@JoyfulNoise & @JamesW) if my CC has a lesser amount of chlorosulfamate than expected. I was preparing for a very slow drop in CC due to assumed chlorosulfamate content, but at this rate, it will take just days to get my FC + CC in check. The pool is crystal clear and shimmering in the sunlight now, which makes me SUPER happy. I will perform the OCLT test in the morning before sunrise to provide a final confirmation that my SLAM is coming to an end. Thanks again to everyone who has chimed in!
It’s difficult to determine exactly when the sulfamic will break down.
When it happens, the pH and TA will suddenly begin to drop and the CC will go away.
When it happens, the pH and TA will suddenly begin to drop and the CC will go away.
Good to know! I'll do an electronic pH test in the morning along with the FC + CC test and report back. Many thanks!It’s difficult to determine exactly when the sulfamic will break down.
When it happens, the pH and TA will suddenly begin to drop and the CC will go away.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

