Hello,
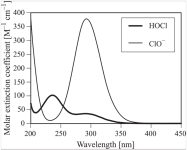
Still interested in the theory and consequences of chlorine protection from UV by CyA, I'm looking for data published in scientific or technical papers relating to measurements concerning this protection.
I have seen, here and there, mentions of such papers as well as curves "based" on measurement results but I haven't been able yet to find and read those reports and the corresponding data.
If you read this post and that you know of such references, would you be so kind so as to let me know where and how to get them ?
Thank you in advance and best wishes to all for the starting new year.
Still interested in the theory and consequences of chlorine protection from UV by CyA, I'm looking for data published in scientific or technical papers relating to measurements concerning this protection.
I have seen, here and there, mentions of such papers as well as curves "based" on measurement results but I haven't been able yet to find and read those reports and the corresponding data.
If you read this post and that you know of such references, would you be so kind so as to let me know where and how to get them ?
Thank you in advance and best wishes to all for the starting new year.