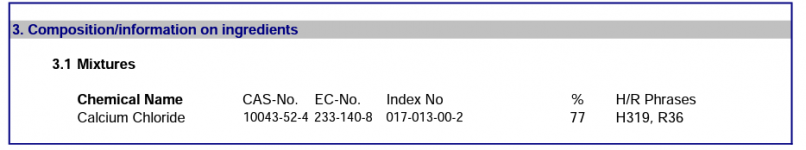
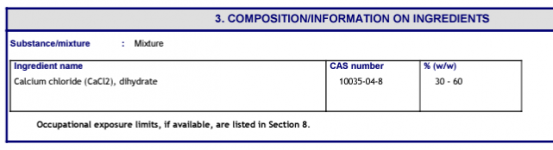
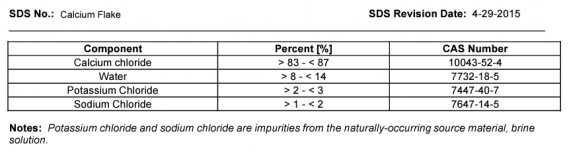
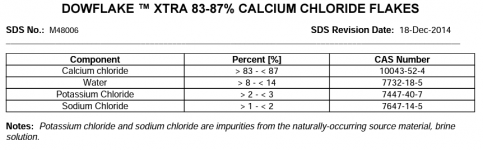
This thread is troubling me a little. for over 20 years, this forum (and others) have NEVER reported this FC loss when adding CH. Now one member reports a halt in FC production on his SWG and another reports a 4.0 ppm loss overnight of FC,
I don't think TFP should suggest any action to counteract this at this point. TFP knowledge thrives on science as well as anecdotal reports from pool owners. Until we can find some repeatable experiments and a scientific reason for it, we should continue our present suggestions for the addition of CH.
I strongly believe TFP needs to stay simple and adding some caveat to CH additions just doesn't have merit.........yet.
I don't think TFP should suggest any action to counteract this at this point. TFP knowledge thrives on science as well as anecdotal reports from pool owners. Until we can find some repeatable experiments and a scientific reason for it, we should continue our present suggestions for the addition of CH.
I strongly believe TFP needs to stay simple and adding some caveat to CH additions just doesn't have merit.........yet.