Looking for some advice on correcting the issues of Calcium build up and the inability to lower the PH. We have moved into a house approximately 18 months ago and have been battling with issues of a calcium problem (assumed) and a constant high PH level.
In summary:
Pool is in Queensland, Australia
My assumption is that the pool was not properly looked after for a long period, hence the calcium issue.
Also, my thoughts are that the high calcium level may be neutralizing the acid, which is probably why I am having trouble dropping the PH level.
I did read somewhere that a possible cure with the calcium build up would be to drop the PH level to 6.8 for a period to get rid of the calcium issue, and as we are going into winter, this is a possibility.
So, I am looking for the advice from the experts on this forum - suggestions/advice please?
In summary:
Pool is in Queensland, Australia
- 45,000 litre saltwater pebblecrete pool
- Yellowing of the surface in patches and around the waterline, which I assume is calcium buildup
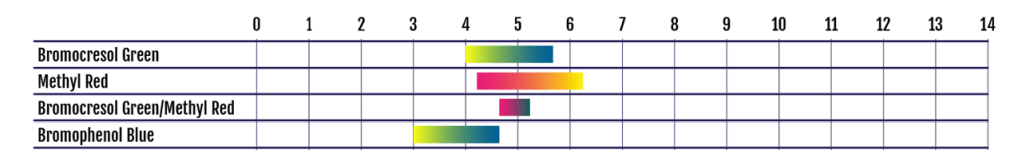
- Constant high PH (8.2 - 8.4) despite adding at least 5 litres of acid (over a 4-5 month period)
- Acid demand 2 drops (equates to need to add approx 400 ml acid??)
- Salt water chlorinator cell has been cleaned but CL level remains around 1.2 - 1.5
- Salt concentration is around 6000 ppm which is the specified level for the brand of chlorinator
My assumption is that the pool was not properly looked after for a long period, hence the calcium issue.
Also, my thoughts are that the high calcium level may be neutralizing the acid, which is probably why I am having trouble dropping the PH level.
I did read somewhere that a possible cure with the calcium build up would be to drop the PH level to 6.8 for a period to get rid of the calcium issue, and as we are going into winter, this is a possibility.
So, I am looking for the advice from the experts on this forum - suggestions/advice please?