New Pool Owner - Testing Myself
- Thread starter Cohnhead
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Gary Davis
Well-known member
As you mentioned, there are a variety of "strengths" of calcium chloride on the market and we all are worried at first about the mystery missing percentage.I don't like the 94% pure. There can be alot of devil in those details. It might be rock salt, it might be iron.
Types of Calcium Chloride
"I see on pool math there are two types of Calcium Chloride. One being Dihydrate and the other I assume is Anhydrous."
Calcium Chloride Concentrations
"Commercial dry Calcium Chloride Concentrations are 74%, 77% (dihydrate), 83-85%, 88-90%, and 94-96% (anhydrous)."
That's a great find of what that mystery ~6 percent is comprised of (actually it was a mystery 3%, almost all of which is table salt).So it's Rocksalt, iron, magnesium, sulfates and some others.
- Product name = Calcium Chloride 94% anhydrous CAS 10043-52-4
- CaCl2 purity = 94% minimum (result 95.6)
- Ca(OH)2 alkalinity = 0.25% max (result 0.13%)
- NaCl alkali chloride = 5% max (result 2.8%)
- Insoluble H2O = 0.25% max (result 0.02%)
- Fe = 0.006% max (result 0.002%)
- MgCl2 = 0.5% max (result 0.25%)
- CaSO4 = 0.5% max (result 0.03%)
- pH = 7.5 to 11.0 (result 8.8)
- Nominal: 0.25% + 5% + 0.25% + 0.006 + %0.5% + 0.5% = 6.506% (with sig figs, that's 6%, most of which is table salt)
- Result: 0.13% + 2.8% + 0.02% + 0.002 + %0.25% + 0.03% =3.232% (with sig figs, that's 3%, most of which is table salt)
- 25 pounds of 94% by weight of calcium chloride x 100.0869 g/mole CaCO3 / 110.98 g/mole CaCl = 21.2 pounds effective calcium (is that math correct?)
- 25 pounds of 97% by weight of calcium chloride x 100.0869 g/mole CaCO3 / 110.98 g/mole CaCl = 21.9 pounds effective calcium (is that math correct?)
- 25 pounds of 100% by weight of calcium chloride x 100.0869 g/mole CaCO3 / 110.98 g/mole CaCl = 22.6 pounds effective calcium (is that math correct?)
I bought mine in the summer for about a dollar a pound for the 94% calcium chloride shipped to my door.Plus, SnowJoe is sold out this moment on amazon with an unknown resupply date.
There's always the discussion of what type of calcium chloride it is though.
- 25 pounds of 94% calcium chloride at about $25 and at 21.2 effective pounds of calcium is about $1.18 per pound of calcium (plus a pinch of salt)
- 25 pounds of 97% calcium chloride at about $25 and at 21.9 effective pounds of calcium is about $1.14 per pound of calcium (plus a pinch of salt)
- 25 pounds of 100% calcium chloride at about $50 and at 22.6 effective pounds of calcium is about $2.21 per pound of calcium (sans the added salt)
calcium chloride vs calcium chloride dihydrate
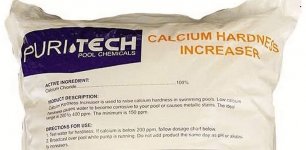
For about two dollars a pound for that Puritech 100% calcium chloride it's twice as expensive, but it doesn't have the 3% to 6% mystery ingredients my shipment had (which appears to be mostly table salt with a pinch of magnesium chloride).I like puritech. It's a few bucks more
I must admit though that I've always been confused by calcium chloride concentrations because I don't have the Richard Falk math figured out for calcium chloride yet. If you have the math figured out such that we can compare the effective dosage in our head like we can for chlorine, please let me know!
Attachments
Last edited:
I must admit that I'm fairly calcium illiterate with a vinyl pool.I must admit though that I've always been confused by calcium chloride concentrations because I don't have the Richard Falk math figured out for calcium chloride yet. If you have the math figured out such that we can compare the effective dosage in our head like we can for chlorine, please let me know!
*also worth noting, both salt and CYA are available in less than 100% concentrations. It doesn't mean they have to have undesirable additions to them. But it certainly means they might. Or that they didn't last time, but next time is another gamble. I personally will not buy any of the 3 that aren't 100%, for one less thing to need to think about.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.