You two understand the FC/CYA relationship and that there is no such thing as chlorine lock where at some CYA level no amount of FC above that is effective. You also understand that you lose less total chlorine at higher CYA levels even with proportionally higher FC levels. You know to decide how much chlorine savings you want vs. the risk of having the higher CYA that would need a much higher FC to SLAM if something went wrong. So this thread is not for you.
What this thread is about is fighting incorrect statements made by some in the industry and those who copy such information as if they were facts rather than looking at the core science and observations to learn the truth. Let's look at each of the graphs in the Kent Williams PPOA article
Cyanurics - Benefactor or bomb? article to see both inaccuracies and deception. Let's start with Figure 1:
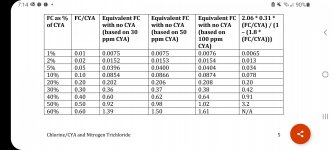
Notice how at 70 ppm CYA the ORP is the same regardless of the FC level. This is an artifact of the ORP system used and has nothing to do with reality in terms of actual active chlorine (hypochlorous acid) level or its effects such as disinfection rate. This is made obvious in Figure 3 which comes from a scientific paper:
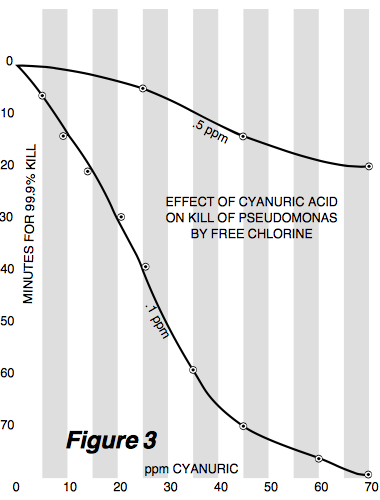
where miracle of miracles look at what happens at 70 ppm CYA. The kill time for 0.1 ppm FC is about 4 times longer than that at 0.5 ppm FC, which is close to what one would expect (it would normally be about a factor of 5 difference since it is roughly proportional to concentration). Let these two graphs and the claims made from each sink in as their points contradict each other. The only explanation is that ORP is in fact useless as an absolute standard, at least for the ORP sensors used for the PPOA paper and for the higher CYA levels. So you can't look at Figure 1 as meaning anything regarding actual disinfection or oxidation rates at higher CYA levels. And as I noted in an earlier post in this thread, if you look at a constant active chlorine level with an FC/CYA ratio of even 5%, then for the 0.5 ppm FC in Figure 1 this would be a CYA of 10 ppm and a reasonably fast 99.9% kill time (from other studies of around 3 minutes but looking like 2 minutes in the graph). The PPOA article is extraordinarily deceitful when it states:
Stated simply, as CYA exceeds 70 ppm, virtually any level of chlorine will result in no more than about .2 ppm equivalent effectiveness. Longer lasting, yes. Better working, No.
when the truth is what the article later states which is:
At much over 50 ppm CYA, controllers are a bad investment too; and as the water approaches 70 ppm CYA, controllers of ORP (the principle behind virtually every pool-chlorine automation device on the market today) simply quit - dead.
It's the ORP sensors that are the problem and fail to operate properly at higher CYA levels. This has NOTHING to do with "equivalent effectiveness" or not being "better working". Lies, lies, and yet more lies. Also, the equivalent effectiveness scale is wrong since 4 ppm FC with 40 ppm CYA and 1.5 ppm FC with 15 ppm CYA are closer to 0.1 ppm FC with no CYA. Also, 700 mV should be closer to 0.1 ppm FC with no CYA equivalent (at pH 7.5), not 0.3 ppm as shown in the graph. Other ORP sensors (e.g. Chemtrol) at least get that equivalence right though this does show that ORP can and does vary by sensor manufacturer.
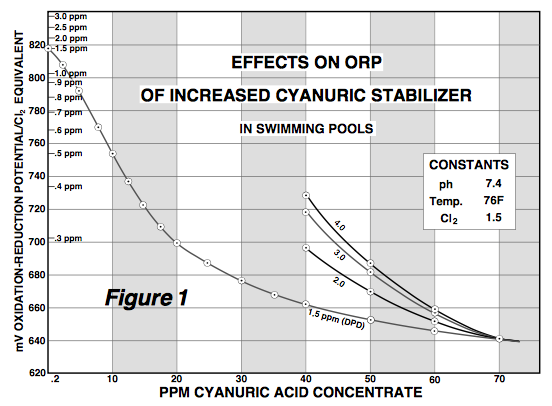
Now let's look at the Chlorine Staying Power (Figure 2 though not labeled as such):
This graph is absolutely positively not true and not at all what is seen in thousands upon thousands of outdoor residential pools. The graph implies significant diminishing returns at higher CYA levels but the degree of such diminishment is not at all what we see, even when we have the FC raised proportional to the CYA level which for the above graph was probably not done (so the graph is even MORE inconsistent with reality). Again, look at Mark's experiments that showed significant increases in chlorine retention at higher CYA levels.
Anyone can tell you that there is no way at 30 ppm CYA that you get (as shown in the graph) 90% of the chlorine remaining after a day of full sunlight. At 30 ppm, one typically loses more than half, nearly two-thirds, of their FC in a day while at 50 ppm this drops to less than half at around 40% loss and at 80 ppm drops to around 20-25% loss and this is all with maintaining a constant FC/CYA ratio for the same active chlorine level. Typical non-SWG pools on TFP at 50 ppm FC use 2-3 ppm FC per day going from 6 or 7 ppm down to 4. That is NOT 90% staying power.