Welcome to TFP!

jpm said:
I'm interested in your ORP conclusions. It seems like ORP is a more effective way to measure whether chlorine is really disinfecting and not bound up by CYA etc. Looking at the OBrien paper there's so many equilibrium reactions that anything could be happening in the water, while ORP seems to measure the available disinfecting power of whatever is in the water.
This is absolutely not true that "there's so many equilibrium equations that anything could be happening in the water". Since when did having simultaneous chemical equations of equilibrium mean that the result was not specific and determinate? There is no question that the calculations are tedious, but they are nothing more than a straightforward application of 1st year college chemistry. See the Pool Equations spreadsheet that precisely calculates the active chlorine (hypochlorous acid) concentration based on pool water chemistry parameters (it also calculates ORP for a few of the different manufacturer's sensors). There are also standard chemical equilibrium programs one can purchase (CHEMEQL, EQS4WIN, MINEQL+, etc.) where one simply enters in the equilibrium constants and initial conditions and out comes an answer. Also see
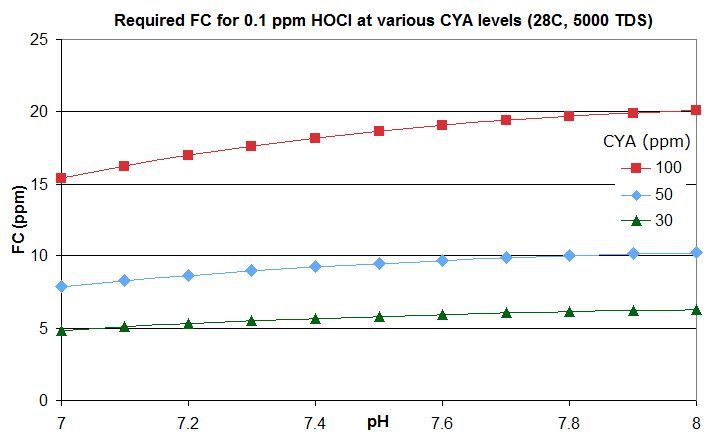
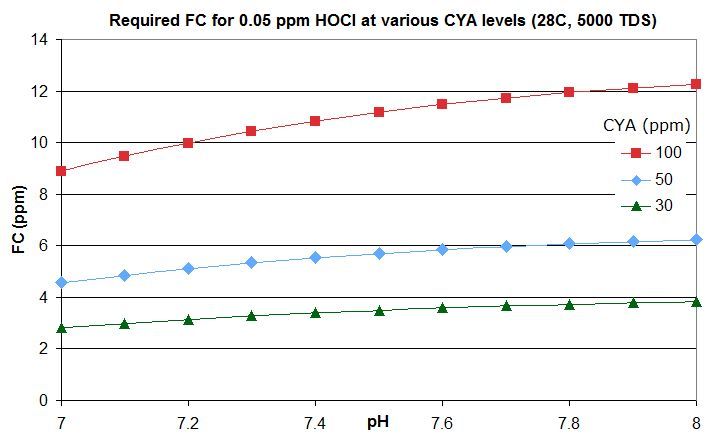
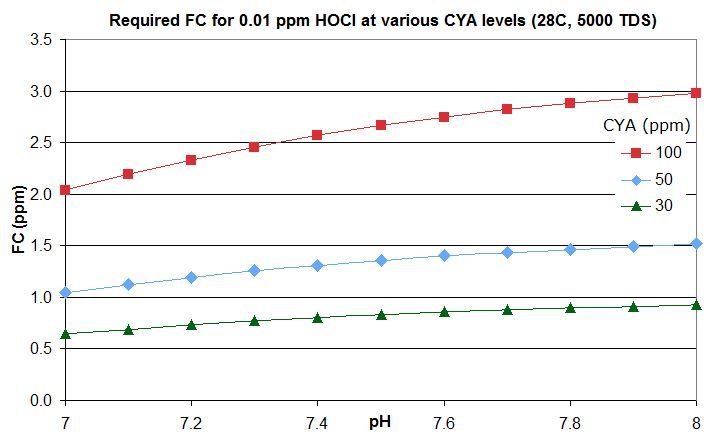
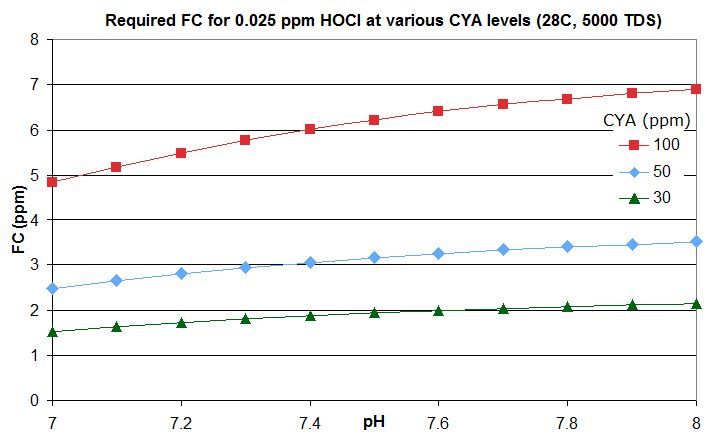
this post in this thread that derives the rough rule of thumb that a pool with Cyanuric Acid (CYA) has roughly the same active chlorine level as a pool with an FC that is FC/CYA when no CYA is present. So a pool with an FC that is around 10% of the CYA level has the same active chlorine as a pool with 0.1 ppm FC and no CYA.
jpm said:
This paper concluded that meeting the minimum pool code did not assure meeting an ORP >= 650 mV.
It also notes that eight states include ORP, and that in Iowa, an ORP < 650 mV could close a pool/spa regardless of other parameters.
http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2646482/
Interesting also that Germany enforces ORP in their swimming pool standards (probably because Europeans have so much more experience with ozone etc.]
The paper was not surprising at all since it is well known that ORP is mostly affected by the hypochlorous acid concentration and not the chlorine bound to CYA. It is true that there is a lot of over-stabilization in some pools. However, if you look at bacteriological field studies, such as the
Pinellas County 1992 (1994) pool study, you find that it takes a very low level of active chlorine to prevent uncontrolled bacterial growth. The issue has more to do with kill times to prevent person-to-person transmission of disease. For residential pools, the issue is more about algae growth since it takes much higher levels of chlorine to kill algae than to kill bacteria (at similar kill rates -- for bacteria you want to kill them a lot faster than they can reproduce while with algae you only need to be somewhat ahead of their reproduction rate).
It is not true that Germany enforces ORP in spite of what the article said. They don't, at least not as a primary determinant. DIN 19643 has specific tests for bacterialogical quality and for FC levels with no CYA in the water. ORP is a secondary measurement. Specifically, 19643 requires a 4-log (99.99%) reduction of
Pseudomonas aeruginosa in 30 seconds. They require 0 CFU in 100 ml for
E. coli,
P. aeruginosa, an aerobic count of 100 cfu/ml and 0 CFU in 100 ml for Legionella. It has a pH range of 6.5 to 7.6 and requires Free Chlorine to be between 0.3 and 0.6 ppm (with no ozone; with ozone the range is 0.2 to 0.5 ppm). The Combined Chlorine (CC) is to be <= 0.2 ppm and TTHM is to be <= 20 ppm. The ORP is 750-770 mV, but this is not the absolute standard since it can vary for other reasons. Nevertheless, given the pH and FC ranges and not having CYA in the water, achieving that ORP is not difficult, but it depends on whose sensors you are using.
jpm said:
In the CDA Model Aquatic Health Code, it seems to confirm that CYA reduces ORP readings, ie. reduces sanitizing power, by actively lowering the ORP of the water. This corresponds to what's known about high CYA lowering disinfection rates.
You are correct that CYA decreases the active chlorine (hypochlorous acid) level and lowers not only ORP but also oxidation and disinfection rates. However, you need to look at this the other way around which is that most pools with no CYA, especially in the U.S. where the FC is 1-2 ppm or more, are OVER-CHLORINATED. They have too high an active chlorine level so oxidize swimsuits, skin and hair too quickly and react chemically with organics to produce disinfection by-products more quickly and to produce more nitrogen trichloride which is very irritating and volatile. The Germans intentionally lowered the FC target to try and minimize these problems, but it is very hard to maintain 0.2 or 0.3 ppm FC everywhere in the pool unless one has high capacity feed systems and outstanding circulation. Another alternative is to use CYA in the water, but in moderation, since CYA is a hypochlorous acid buffer. So one can have 4 ppm FC with 20 ppm CYA for the equivalent of 0.2 ppm FC with no CYA but with an ample reserve of chlorine nearly instantaneously available wherever it is needed to meet local chlorine demand.
My wife has personal experience with this over-chlorination problem where her swimsuits would degrade every year during the 5-month winter swim season when she swam in an indoor community center pool with 1-2 ppm FC and no CYA 3-4 times a week. In our own pool with 3-6 ppm FC with 30-40 ppm CYA during the 7-month swim season swimming nearly every day, her swimsuits last for around 7 years. The difference is that our pool has the equivalent FC of 0.1 ppm with no CYA so that the indoor pool is 10-20 times higher in active chlorine level and degrades her swimsuits much, much faster (and she reports her skin is flakier and hair frizzier when using that pool).
I went to the World Aquatic Health Conference (WAHC) October 11-12th and spoke to those associated with the CDC Model Aquatic Health Code (MAHC) regarding their desire to eliminate CYA entirely from indoor pools and spas and high-risk venues. They are reconsidering this since it is an extreme reaction to fears of not being able to deal with the protozoan oocyst
Cryptosporidium parvum that has consequences, especially when retaining the higher FC levels. By limiting the CYA level, not eliminating it, one can still shock to kill Crypto and there are other methods such as using chlorine dioxide that might be easier.
jpm said:
A portable ORP tester might be an "inexpensive" investment - $100 will get you a Hanna ORP/pH/temp meter on Amazon. The Oakton meter (used in the study) seems to go for $200, ORP only, but doesn't seem to need as much calibration and storage solutions. They don't say how long the electrodes last, so could be pricey unless you can get several hundred tests out of them. As a bonus, the meter would tell you if your ozonator is working or contributing.
If you looked at the
ORP post and the links to manufacturer's own tables of ORP vs. FC in various conditions, it's all over the map. That means using ORP as a reliable measure of disinfection isn't very useful except on a broad scale. The ORP data is all over the place. Also, as for the Oakton meter, it was used in a study of 194 pools taking 620 samples as shown in
this post. Note how the ORP is much more highly correlated to the calculated hypochorous acid concentration (which is roughly half the FC/CYA ratio) than it is to FC alone. This is, of course, expected, yet the 2009 paper you referenced didn't even bother doing the calculations for HOCl. Also note that in the pool study using the Oakton meter, many pools also had built-in ORP systems yet in 23% of those measuring the same water they showed 100 mV or more differences, some higher some lower. This wasn't just an issue of calibration either -- the Oakton was regularly calibrated for each day's measurements.
ORP measures a thermodynamic quantity, not reaction rates which is really what is relevant, and even then it doesn't actually follow the Nernst equation for chlorine and no one has explained why. The closest fit implies a chemical reaction with 0.6 to 0.8 electrons instead of the expected 2-electron reaction. Also, many chemicals including dissolved hydrogen gas from saltwater chlorine generators or the use of non-chlorine shock (potassium monopersulfate) can all affect ORP readings but do not result in the corresponding implied changes in disinfection rates. At best, ORP can be used for process control, as a setpoint, when accurately measuring FC, CYA and other water chemistry parameters.
Why spend hundreds of dollars on an ORP sensor when given the FC and CYA at normal pH near 7.5 you will know the active chlorine level? All you have to do is choose your FC/CYA ratio for your purposes. For those on this and other pool forums, the goal is to have enough chlorine to kill green and black algae faster than they can grow. In manually dosed pools, this is roughly an FC that is 7.5% of the CYA level (in saltwater chlorine generator pools it's roughly an FC that is 5% of the CYA level). If you were wondering what the kill times are like,
this post lists them for an FC that is around 10% of the CYA level -- the equivalent of 0.1 ppm FC with no CYA. For commercial/public pools, a decent minimum would be no lower than this and might be an FC that is 20% of the CYA level so equivalent to 0.2 ppm FC with no CYA. This balances disinfection and oxidation times against minimizing disinfection by-products and oxidation of swimsuits, skin and hair and of corrosion rates.