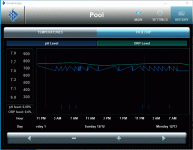
Preface: I understand that UV light lowers the ORP of FC due to the interaction of CYA and Chlorine. I've read most of chem geek's threads on this topic, and I have observed this behavior directly.
Question: How does this UV-CYA-FC interaction affect pH? What I have observed is that, during the course of the day, pH will drop following the same curve as the ORP during the day and rise again during the night. Hopefully someone here can shed some light on this.
Background: I have a functioning, relatively stable ORP/pH control system implemented on my pool. To achieve this has taken the better part of 5 years, but I have managed to work out 99% of the challenges introduced by having an outdoor pool in the extreme environmental conditions of the Arizona desert. (I'd be happy to share this story, but perhaps on another thread). I first noted the pH changes when I was running the control system 24hrs/day. I noticed that the acid usage always increased during night hours and my TA usage was disproportionately high. I assumed (wrongfully) that it was simply a reaction to the introduction of a strong base (Sodium Hypochlorite) during the daytime. Later, when I realized that running the control system 100% of the time was not needed, I also noted that my acid and TA usage went way down with no change in the pH stability. I started to look carefully into this by tracking the diurnal curves of both ORP and pH (with no Chlorine or Acid additions) and was able to confirm this behavior.
Thank you in advance for any help/insight you may have!
Robert
Question: How does this UV-CYA-FC interaction affect pH? What I have observed is that, during the course of the day, pH will drop following the same curve as the ORP during the day and rise again during the night. Hopefully someone here can shed some light on this.
Background: I have a functioning, relatively stable ORP/pH control system implemented on my pool. To achieve this has taken the better part of 5 years, but I have managed to work out 99% of the challenges introduced by having an outdoor pool in the extreme environmental conditions of the Arizona desert. (I'd be happy to share this story, but perhaps on another thread). I first noted the pH changes when I was running the control system 24hrs/day. I noticed that the acid usage always increased during night hours and my TA usage was disproportionately high. I assumed (wrongfully) that it was simply a reaction to the introduction of a strong base (Sodium Hypochlorite) during the daytime. Later, when I realized that running the control system 100% of the time was not needed, I also noted that my acid and TA usage went way down with no change in the pH stability. I started to look carefully into this by tracking the diurnal curves of both ORP and pH (with no Chlorine or Acid additions) and was able to confirm this behavior.
Thank you in advance for any help/insight you may have!
Robert