Interesting. That said, I never use the
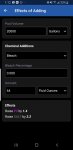
Effects of Adding for calculating my chlorine additions. Doing so is guess and check, and pointless when it has a section for calculating the special amount of chlorine to add. And this seems to work as expected for any concentration of bleach.
For example, to raise 2.5 ppm with 6% requires 104 oz.
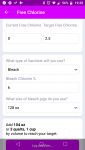
If I change to 3% it's 214 oz, not quite but very very close to 2x as much, and 12% calculates out to 64 oz.
If you type these values into effects of adding, only the 6% calculates out to 2.5 ppm. The 12% numbers work out to 2.1, and the 3% say 4.5 ppm. So it appears that the Chlorine addition section works linearly with %, but not the effects of adding.
Annoyingly, I've sometimes noticed a bug in the chlorine additional section, where it will sometimes give an incorrect value for the amount to add. Usually I spot this when using in real life, and it's rare. It happened a LOT more trying to write this post with these demo numbers, and I tried, and failed, to tack down a specific, repeatable sequence of events that caused it, so it could get fixed. When it happened instead of telling me 104 oz of 6% I'd get something like 124 oz, but if I would change a value and change it back it would then say 104 oz again. I've seen this enough there's an actual bug, but until I (or someone) can nail down the exact sequence it'll be tough to fix.