I can answer in detail later tonight. But basically here's the run down -
FC/CC Test - Uses DPD dye for a pink color and ferrous ammonium sulfate as the titrant. Dye is oxidized by FC to a Wurster dye to turn pink and the FAS converts it back. Potassium iodide is then added which causes the CC's to oxidize the dye and then more FAS is added to titrate. Hach has a very good PDF on the reactions.
TA - this is just a titration of the alkalinity in the pool water. Basically a specialty dye is added to the water sample with a green/red transition at a pH lower than 4.5. Sulfuric acid is the titrant where each drop neutralizes approximately 10ppm of alkalinity (carbonates, borates and cyanurates). Once the pH drops below 4.5, the test is complete.
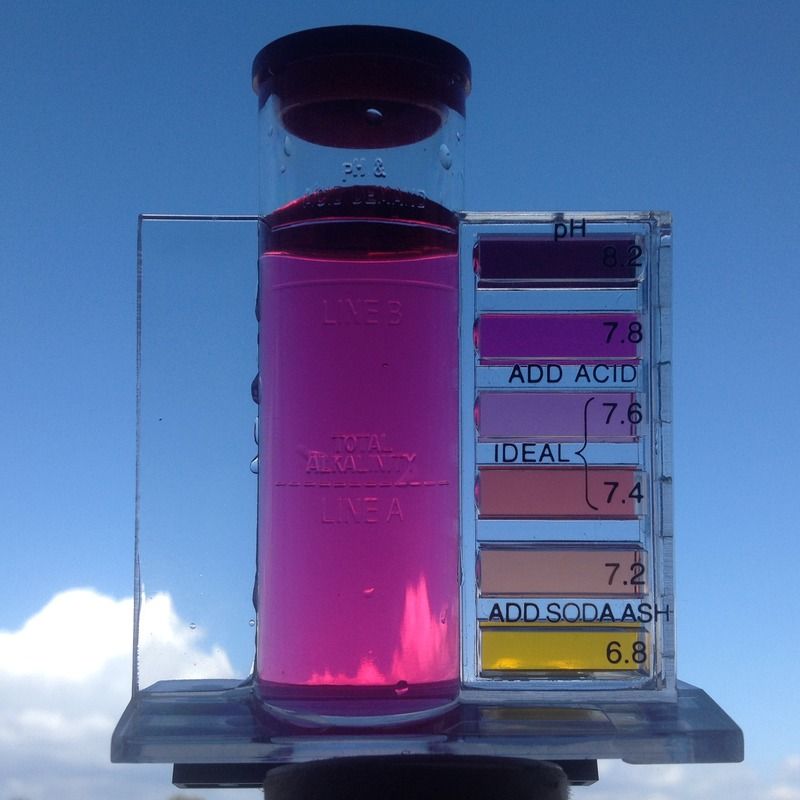
pH - just phenol red indicator with a mix of dechlorinating chemicals
CYA - melamine is the R-0013 reagent. It reacts with CYA at low pH to form a melamine cyanurate precipitate (actually a hydrogen bonded melamine cyanuric acid adduct). The test is basically a crude turbidity measurement.
CH - Eriochrome Black dye is the indicator. R-0010 is just sodium hydroxide to reduce the magnesium concentration (by forming magnesium hydroxide). The R-0012 (eriochrome black) reacts with divalent metals (Ca2+) to form a reddish-pink complex. The titrant is a mixture of EDTA and another chelant that more strongly attracts the calcium ions. Once all of the calcium is stripped form the eriochrome black, the dye changes from reddish-pink to blue.
Salt - Mohr's argentometric titration. Potassium chromate is the yellow-red/brown indicator. Silver nitrate is used to precipitate out silver chloride. Once all of the chloride is removed from the solution, the silver reacts with the chromate to form a reddish-brown silver chromate compound.
Those are there major tests. They can be found in just about any quantitative chemistry or solutions chemistry textbook you might have access to.



 Ar yes, sorry that's me just thinking about myself again. With lots of rain I've only every had low calcium and keep it up to prevent the water leaching calcium from the concrete. High calcium is just as important and pool school deals with CH.
Ar yes, sorry that's me just thinking about myself again. With lots of rain I've only every had low calcium and keep it up to prevent the water leaching calcium from the concrete. High calcium is just as important and pool school deals with CH.