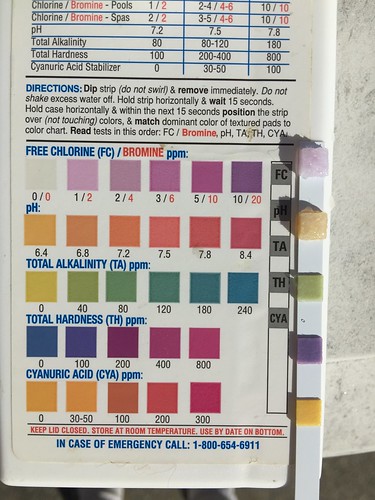
Just opened my pool, and it looked fairly good, just slightly cloudy. Put some chlorine in and turned on the pump and came out to a pool the next morning that was very cloudy the next morning and I could not see the bottom. Added more chlorine and checked and found pH down around 6.4. I had lost my heatpump at the end of last season and it had dumped all the Freon R22 and oil into the water - yuk. So besides adding more chlorine I added two boxes of Borax (20x40 IG pool). That brought the pH up to about 6.6 so I dumped the other two boxes of Borax I still had in and it is sitting at 6.9 right now. Dumped a pound of shock and two large bottles of Chlorox in - that is all I had on hand this afternoon. I am very perplexed at the blue color on the filter of my robot as I have never seen this color blue in the pool before. I had some sediment in the little jar that lets you see what is going to waste when you backwash the filter and I poured it out onto an old white table. It is very fine blue material. Any clues what this might be or how to clean it up. Pictures below: