Re: Total Control - ORP vs. Chlorine PPM - Confused
Thanks

>>and that means they will be generating hydrogen gas bubbles for a lot longer and of course generating chlorine gas a lot longer
I also thought about that. But i think the proportions of generated hydrogen and generated Cl² should be always the same? No matter if the cell is running 1hr/day or 10hr/day. So it maybe should affect the ORP readings the same way all over the day?
>>Basically at the higher output, the bubbles may coalesce more and outgas more. With lower output, they remain smaller and distinct longer and therefore can dissolve better.
hm... this is perhaps a possibility. But to get back to "my" chemist: He did a calculation for that last year (I have no numbers) but he said that it would take such an amount of free chlorine to oxidize the hydrogen that there wouldn't be any FC left in the water when hydrogen would really dissolve.

>>Do you notice any correlation with flow rate through the SWG?
No, not really. Some of the people have a Speck Eco Touch installed (similar to the Pentair intelliFlow) and there is no visible (or assignable) difference if the flow rate is at about 4m³/h (1 gallon) or at 12..15m³/h (3-4 gallons/hr)
>> Finally if there were more of a problem in pools with still water compared to those with aeration features (waterfalls, spillovers, fountains, etc.) then that would also be consistent with some sort of gas transfer.
Unfortunately, most of the pools in Germany don't have any of these gadgets

... there is only one guy (with this "ORP problem" ) who has a hydro-flow system installed and if he starts it, ORP level is in deed rising (reproducible). But there is only this single pool, so I can not say whether this is a general behaviour or something like an artifact.
>> Adding hypochlorite raises the pH so you should first see if just raising the pH also resolves the problem.
No, rising the pH only, lowers the ORP level (as expected)... if you already have ORP levels at about 100mV you don't really see any more changes in ORP, but if the ORP is at a more normal level you can see it falling if you only rise pH with sodium hydrogen carbonate (NaHCO3).
>>What is interesting is that if you add some hypochlorite, the problem goes away.
yes, interesting

...this is a typical example of what we see here in this case:
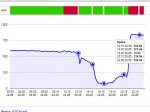 http://www.lembi.de/pd/shareX_54635204.jpg
http://www.lembi.de/pd/shareX_54635204.jpg
Pool: 32m³ of water (8460 gallons), pH 7.0. CYA 0, TA normally between 20 and 40 ppm, Cell production rate: 11g/hr (a small one)

The green bar above the graph shows the working cell. SWG was running nearly all over the night and you can't see a real change in ORP level. He had a DPD reading of about 5.5ppm FC in the morning (way too much), opened up the cover at 12:00 (sunny day, water at about 25°C ... 77°F). You can see the ORP falling from that point till it completely collapses (60..100mV). It starts rising again in the afternoon, when UV intensity is weaker, but if you do "nothing" in most of the times you are not able to raise it back to 600..700mV only using the SWG. DPD reading at 8:00pm was 0.8ppm. He added 80 gram CH at about 9 pm which should raise FC (HOCl) level for about 1.5 ppm. Not too much, but enough to get back an ORP level of 830-850 which is absolutely OK/normal for that FC levels and clean water. pH of course also reacts and goes up for a short period, but the dosing control brings it back down to the setpoint within 45..60 minutes.
As I mentioned previously, this is absolutely reproducible (not only in this basin).
>>One obvious question: where did you put your ORP system? I assume you didn't put it after the SWG and instead have it somewhere in the circulation system before the SWG, correct?
Yes, sure

. The probes - more precisely the measuring cell - is always installed right after the filter, the SWG and the acid injection are always the last parts in the system, right before the water gets back into the pool. In most cases pH injection is the last part and SWG is before that (depending on how the users installed their system).
>> So what does your ORP vs. FC standard look like
For the pools with liquid dosing we recommend a setpoint anywhere between 770 and 800mV (0 CYA, pH7.0). This works for 90% of the pools quite well with resulting FC values between 0.6 and 1.0 (of course depending on temperature an pH value which is set to 7.0 in most cases). Sure there are some exceptions (in both directions) and some have ~740mV at 1.0ppm and some have 850mV at 1.0ppm but the majority is really between 770 and 800mV and dosage is working really reliable.
>>but isn't so good for determining the FC level even holding other parameters fixed
Yes, of course you are not able to hold a fixed FC level of - let's say - 0.6ppm. But under normal conditions it will result (on a sunny day) at a lowest value of about 0.3/0.4ppm (early afternoon) and a highest value of 0.8 / 0.9ppm in the evening. If you have guests and 20 people used the pool, it surely will result in FC levels of about 1.5 or up to 2.0ppm in the evening. But actually i think that's ok in this moment and fits the demand of more HOCl to bring the water back to a "clean condition" and reduce CC back to a lower level.
Maybe even the better way for residential pools than holding a fixed FC value of 0.4 ... 0.6 ppm (0 CYA) because the system will always raise the HOCl level to a point where (depending on the pollution of the water) a safe disinfection is guaranteed...
...as long as the measurement works

. But it does - without any problems - for liquid dosing, but not for 60-70% of the pools using SWG.