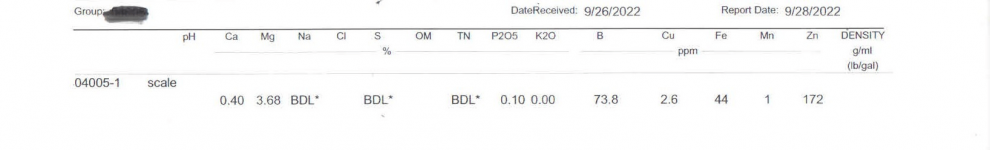
With the scale Sulfur level at BDL, reviewing the pool water results and the reaction from my quick MA test on the scale, my best guess would be HCO3.
Get a SWCG, "it will be great" they said......
- Thread starter Oly
- Start date
You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
The whole point of a test is for them to tell you what the scale is composed of.
They have identified less than 5% of the composition of the scale.
I don’t see the point of guessing when the lab should be telling you what the composition is.
Are they saying that 95% is of unknown composition?
They have identified less than 5% of the composition of the scale.
I don’t see the point of guessing when the lab should be telling you what the composition is.
Are they saying that 95% is of unknown composition?
You won't get solid calcium bicarbonate or magnesium bicarbonate.my best guess would be HCO3.
You can get calcium carbonate or magnesium carbonate.
If it's carbonate, why isn't carbonate reported?
If it's carbonate, what is it combined with?
A carbonate would usually be combined with magnesium or calcium and these are reporting very low.
The calcium is at 0.40% and the magnesium is at 3.68%?
Last edited:
- May 23, 2015
- 24,382
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
If this is an AG lab, then I suspect their analysis is going to be very simple and isolated to only those components that are applicable to soil testing. This isn't a forensic lab or a chemistry lab that has access to high end analysis equipment. I agree that the test results look off, but it could just be the way in which they typically report results for soil analysis.
The scale is almost always a mixture of calcium and magnesium carbonate. Sometimes magnesium hydroxide forms as that is also fairly insoluble. Most magnesium scale compounds are mushy and soft. Calcium scale tends to be more adherent and hard. If sulfates and/or phosphates are present, you'll get the formation of calcium sulfate (gypsum) and calcium phosphate (apatite). Phosphate scales are the worst because they are hard, adherent, and almost completely resistant to acids. The high zinc levels are unusual and suspect given that you have no zinc components in your pool. Even so, zinc is very unstable in water and almost always forms hydroxide and oxide precipitates.
The scale is almost always a mixture of calcium and magnesium carbonate. Sometimes magnesium hydroxide forms as that is also fairly insoluble. Most magnesium scale compounds are mushy and soft. Calcium scale tends to be more adherent and hard. If sulfates and/or phosphates are present, you'll get the formation of calcium sulfate (gypsum) and calcium phosphate (apatite). Phosphate scales are the worst because they are hard, adherent, and almost completely resistant to acids. The high zinc levels are unusual and suspect given that you have no zinc components in your pool. Even so, zinc is very unstable in water and almost always forms hydroxide and oxide precipitates.
Why are the calcium and magnesium levels so low according to the report if they are going to be a part of most or all scale?
It seems to me that the levels would be much higher if they were part of all scale.
They total 4.08% of the total weight of the scale.
It seems to me that the levels would be much higher if they were part of all scale.
They total 4.08% of the total weight of the scale.
- May 23, 2015
- 24,382
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Why are the calcium and magnesium levels so low according to the report if they are going to be a part of most or all scale?
It seems to me that the levels would be much higher if they were part of all scale.
They total 4.08% of the total weight of the scale.
I agree. That does not make sense. The analysis of the scale is either incomplete or incorrect. I was merely stating what the scale ought to be, based on the pool water chemistry.
Thanks James, I will work with the lab mgr and dig a bit deeper....
On day four now of cell operating as designed.
Matt, Ag labs focus on soil, water, plant tissue and fertilizer tests typically. They should have the equipment to get a more detailed analysis.
On day four now of cell operating as designed.
Matt, Ag labs focus on soil, water, plant tissue and fertilizer tests typically. They should have the equipment to get a more detailed analysis.
Magnesium carbonate can be anhydrous (MgCO3), magnesium carbonate dihydrate (MgCO3•2H2O), magnesium carbonate trihydrate (MgCO3•3H2O) or magnesium carbonate pentahydrate (MgCO3•5H2O).
The water will lower the amount of magnesium by weight percent, but the amount of magnesium and calcium still seem like they are too low as a percentage of total weight.
The water will lower the amount of magnesium by weight percent, but the amount of magnesium and calcium still seem like they are too low as a percentage of total weight.
- May 23, 2015
- 24,382
- Pool Size
- 16000
- Surface
- Plaster
- Chlorine
- Salt Water Generator
- SWG Type
- Pentair Intellichlor IC-60
Thanks James, I will work with the lab mgr and dig a bit deeper....
On day four now of cell operating as designed.
Matt, Ag labs focus on soil, water, plant tissue and fertilizer tests typically. They should have the equipment to get a more detailed analysis.
When I was a working man, one aspect of my engineering job was to do some reverse-engineering and forensic chemistry & materials analysis work. We had access to some of the most sophisticated scientific equipment on the planet - optical microscopy with fluorescent analysis, electron microscopes, transmission electron microscopes, X-ray photoelectron spectroscopy tools, Auger electron anlyzers, X-ray diffractometers, mass spectrometers, inductively coupled plasma chromatography, NMR equipment, aqueous chemistry tools, etc, etc, etc. Heck, there was even a time when I could send samples to Brookhaven National Labs to be analyzed using their high-energy beam lines or do neutron diffraction experiments. It was lots of fun to be handed a completed puzzle and slowly take it apart piece by piece.
But there was one thing that every analysis job required and it was absolutely essential to a successful outcome - team meetings. Before any job was ever started, there had to be multiple meetings upfront to discuss what it was we were being handed and to brainstorm ideas for analysis and what we thought we would need to do to complete the job. If anyone blindly handed me a sample and said, "tell me what this is!" I would hand it back to them and say, "No." ... So, definitely get back together with the Ag Lab folks and let them know exactly what you have given them and what you want to get out of the analysis. Knowing what the customer is looking for narrows the potential avenues of investigation and can make the analysis job a lot more thorough and precise.
The P2O5 reading might indicate magnesium phosphate pentahydrate as part of the scale.
Mg3(PO4)2•5H2O
Since the scale seems to be reduced with a reduced phosphate level, magnesium and/or calcium phosphate scale seems likely.
Mg3(PO4)2•5H2O
Since the scale seems to be reduced with a reduced phosphate level, magnesium and/or calcium phosphate scale seems likely.
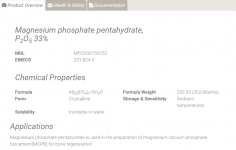
10233-87-1 - Magnesium phosphate pentahydrate, P<sub>2</sub>O<sub>5</sub> 33% - Magnesium phosphate, tribasic pentahydrate - 18219 - Alfa Aesar
Alfa Aesar is a leading manufacturer and supplier of research chemicals, pure metals and materials for a wide span of applications.
Magnesium phosphate pentahydrate (33% P₂O₅)
Magnesium phosphate pentahydrate (33% P₂O₅)
CAS: 10233-87-1 EINECS: 231-824-0 Synonyms: Magnesium phosphate, tribasic pentahydrate
Last edited:
Maybe you're getting an excessive amount of oxygen created and maybe it is combining with unoxidized phosphorous to create phosphorous pentoxide.
I did test my tap water with the K-1106 at 250ppm PO4. I have researched some info that municipal water systems can use phosphates to form a protective scale and protect pipes from corrosion. I also recently used the Orenda PO4 removal product. No known contamination from fertilizers. Grandkids do use zinc oxide sun screen. Some people use body lotions and DET. Leaves, dirt, worms, pollen, insects and even bathing raccoons have found my pool water in the last several seasons.
Yes going into a lab, handing them a sample and requesting to know exactly what it is can be a costly experience. A little direction goes a long way, so thanks for the possibilities. Looking at this from a perspective of my chemistry correction options I may benefit from another round of P removal, but reducing the Mg, Cu, Zn or TDS will require water replacement. The goal of actually identifying the scale type should be tempered by the best (least expensive, practical and long term) solution. The scale testing 0.1ppm P2O5 is 1000ppm and I know my water even after using the Orenda PR-10000 still tests above 500ppm PO4 so this may be the water chemistry river I travel as it really is the only option short of a drain and refill. I will also send the cell into CPool this winter for testing and evaluation.
Yes going into a lab, handing them a sample and requesting to know exactly what it is can be a costly experience. A little direction goes a long way, so thanks for the possibilities. Looking at this from a perspective of my chemistry correction options I may benefit from another round of P removal, but reducing the Mg, Cu, Zn or TDS will require water replacement. The goal of actually identifying the scale type should be tempered by the best (least expensive, practical and long term) solution. The scale testing 0.1ppm P2O5 is 1000ppm and I know my water even after using the Orenda PR-10000 still tests above 500ppm PO4 so this may be the water chemistry river I travel as it really is the only option short of a drain and refill. I will also send the cell into CPool this winter for testing and evaluation.
Thread Status
Hello , This thread has been inactive for over 60 days. New postings here are unlikely to be seen or responded to by other members. For better visibility, consider Starting A New Thread.

