This graph clarified a lot of ideas I had about Cl chemistry in pool water -
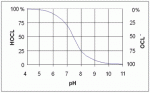
Basically it shows what the make-up of your free chlorine is with respect to pH. Chlorine in water is a balance between hypochlorous acid (HOCl) and the hypochlorite anion (OCl-). Or, in HS chemistry speak -
HOCl <--> H+ + OCl-
Since hypochlorous acid is the more potent germicide than hypochlorite (household bleach is sodium hypochlorite), you want a lower pH environment in order to shift the balance of the equation towards the left side. When water becomes too alkaline (pH near 8.0), your free chlorine is dominated by the hypochlorite species and is therefore less effective.
With that said, one cannot just go to extremes in pH as other factors will dominate (low pH damages pool liners and equipment and high pH causes severe Ca scaling).
Just thought I'd share the above for those who get geeked-out about chemistry....

Basically it shows what the make-up of your free chlorine is with respect to pH. Chlorine in water is a balance between hypochlorous acid (HOCl) and the hypochlorite anion (OCl-). Or, in HS chemistry speak -
HOCl <--> H+ + OCl-
Since hypochlorous acid is the more potent germicide than hypochlorite (household bleach is sodium hypochlorite), you want a lower pH environment in order to shift the balance of the equation towards the left side. When water becomes too alkaline (pH near 8.0), your free chlorine is dominated by the hypochlorite species and is therefore less effective.
With that said, one cannot just go to extremes in pH as other factors will dominate (low pH damages pool liners and equipment and high pH causes severe Ca scaling).
Just thought I'd share the above for those who get geeked-out about chemistry....

