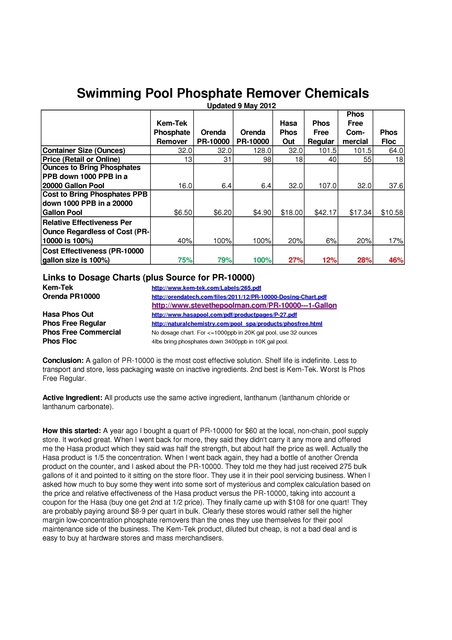
Re: How to calculate cost per effective chlorine liquid v po
Oh. I see. I think. Thanks for the clarification. There is dichlor (anhydrous) and there is dichlor (dihydrate).
From now on, I'm never buying a dihydrate that doesn't say on the box the available chlorine because if they don't say it, I will assume it's not good (and that they're trying to hide it).
The only confusing thing left on that topic is how both a 99% & 85% dihydrate can both have 55% available chlorine?
1. Sodium Dichloro-s-Triazinetrione, anhydrous, typicall 99% by wt, available 64%
2a. Sodium Dichloro-s-Triazinetrione Dihydrate, typically 99% by wt, available 55% <== if this has 55% available chlorine ...
2b. Sodium Dichloro-s-Triazinetrione, typically 85% by wt, available 55% <== how can this have 55% available chlorine?
As a related aside, one of the arguments of the Costco 4-in-1 US Patent 5,670,059 is that the shock is not an oxidizer for transportation purposes.
chem geek said:These two are the same.rock said:- Sodium Dichloro-s-Triazinetrione Dihydrate, typically 99% by wt, available 55%
- Sodium Dichloro-s-Triazinetrione, typically 85% by wt, available 55%
Oh. I see. I think. Thanks for the clarification. There is dichlor (anhydrous) and there is dichlor (dihydrate).
From now on, I'm never buying a dihydrate that doesn't say on the box the available chlorine because if they don't say it, I will assume it's not good (and that they're trying to hide it).
chem geek said:if you find % Available Chlorine as 55% or so (56%), then you've got nearly pure Dichlor dihydrate which is good.
The only confusing thing left on that topic is how both a 99% & 85% dihydrate can both have 55% available chlorine?
1. Sodium Dichloro-s-Triazinetrione, anhydrous, typicall 99% by wt, available 64%
2a. Sodium Dichloro-s-Triazinetrione Dihydrate, typically 99% by wt, available 55% <== if this has 55% available chlorine ...
2b. Sodium Dichloro-s-Triazinetrione, typically 85% by wt, available 55% <== how can this have 55% available chlorine?
chem geek said:Dichlor dihydrate ... is a Class 1 oxidizer as opposed to the anhydrous Dichlor which is a Class 3 oxidizer
As a related aside, one of the arguments of the Costco 4-in-1 US Patent 5,670,059 is that the shock is not an oxidizer for transportation purposes.
patent application said:the combination of three components yields a safer composition than for certain of the individual components, such as the chlorine source material. For example, sodium dichloro-s-triazinetrione is classified as an oxidizer per DOT regulations. This classification indicates certain levels of safety risks and transportation constraints. By contrast, clarifier products formulated based on this disclosed invention have been found to be non-oxidizers by DOT test, which carry fewer safety risks and transportation limitations.