JoyfulNoise suggested I must have non-zero CYA for FC loss to be so low, but, I don't have a way to know what my CYA level really is. So, I don't have a way to get the FC loss versus CYA at very low CYA levels. Since it's so difficult to get that data, I asked if it had ever been empirically verified.
Dave,
I was mistaken because you said you were adding "shock" weekly to your pool in the beginning and I thought it was dichlor shock but it turns out that you were adding cal-hypo. So my assumption that you must have had a low level of CYA was wrong.
Also, let's keep in mind that your pool
is very different than most TFP pools. You run a copper ionizer system with a low level FC. The original method of sanitation you were told to use was to have the ionizer on generating copper all the time and then to just "shock" weekly to get some FC into the pool. But, you surmised that that didn't seem like a reliable method so you added an IntelliChem system to your pool which doses the FC based on ORP feedback (and also actively controls pH). This way you maintain a more consistent FC level rather than the "shock weekly" method. That's certainly beneficial, but not bullet-proof.
First, to address the question you had specifically - no, there is no way to measure CYA less than 20ppm because the test simply cannot read lower than that due to the inability of the melamine-cyanurate complex to form precipitates when the concentration of CYA is less than 20ppm. Even at 20ppm it is very difficult to get a good reading (although members do have some success with it). There are no other chemical methods for testing CYA at low levels so you would have to use a spectrophotometer method (probably something like a LaMOtte ColorQ) to get lower readings although I have no idea if that system is reliable at low levels.
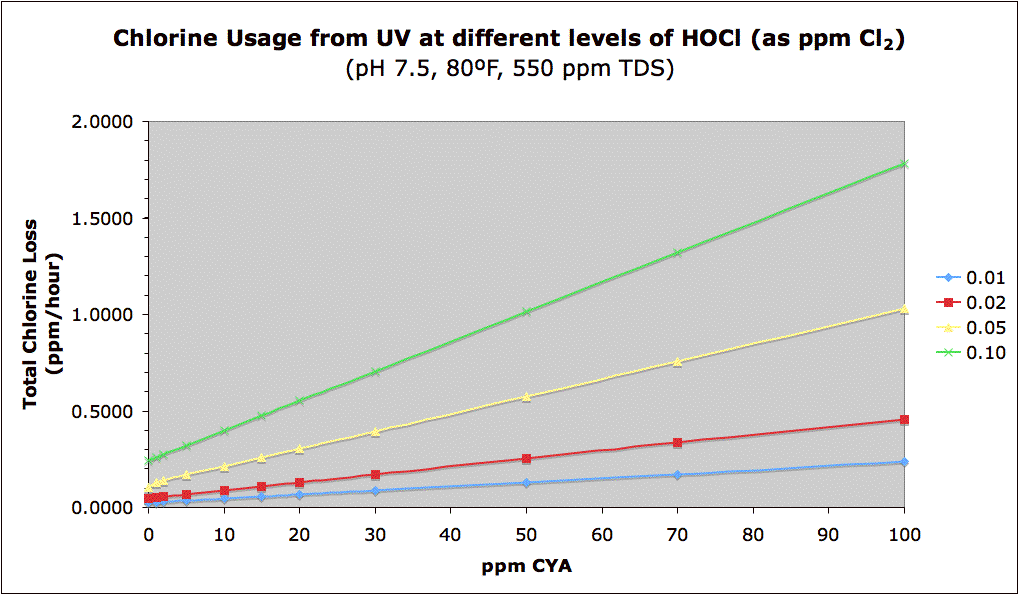
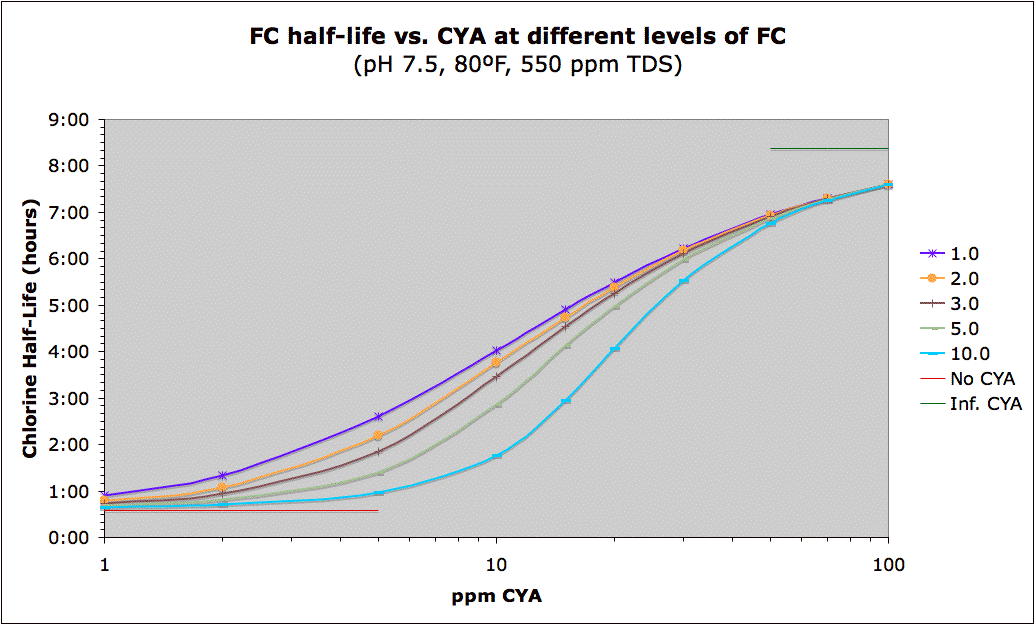
The data is those charts by
chem geek are all, from what I know, theoretically derived. There is no actual experimental data there. You are, of course, welcome to e-mail him (his external e-mail is linked to his profile) to get the exact details of how he derived most of those charts. However, even though the data is derived from the chemistry, it has been reliably matched up to experience.
As for your original post, I don't disagree with what you are seeing. In fact, I think you are confirming the low loss rates at the low end of the chart. It is true that when you increase CYA you increase loss rate, but try to understand why - it is because when you use CYA
you need to have higher FC in order to maintain a hypochlorous acid level above the threshold that will kill pathogens. The first chart you sighted is nothing more than the loss rate one would expect to have when you include the UV loss rates of hypochlorous acid (HOCl), hypochlorite anion (OCl-) and chlorinated cyanurates (HCY-Cl). So if your FC is higher, then your concentration of all of those species is higher and your loss rate is greater (those are the isolines in the graph, essentially lines of constant FC/CYA ratio). The point of the graph is this - if you jump from one isoline to another, say increase your FC/CYA ratio, then your loss rate goes up. So essentially it tells folks this - it's best to keep your pool at an FC/CYA ratio of 5% than it is at 10%. Some people think, "Wow, if I just jack up my chlorine really high, then I can just sit back and not worry about it for a week or so...." But what that chart is telling us is that idea is false - if you increase your FC to really high levels without increasing your CYA (and thus jumping from a lower isoline to a higher isoline), you will make your chlorine loss rates worse (faster). The chart is designed to do away with the notion that "a little is good therefore a lot is better!"
In your case, you have no CYA in your pool and you keep your FC at extraordinarily low levels. Therefore, your loss rate is lower simply because there is less chlorine around for you to lose. Now your ORP probe will add chlorine on-deamnd which is great and it likely keeps your levels stable. But the main problem that I see with your setup is that your levels are so low, and it is a fact that water circulation in all residential pools is terrible ineffective, that it would be easy for your ORP sensor to think that your water is fine when, in fact, there are probably dead spots in the chlorine. Despite what we might think of our pools, there can easily be stagnant areas of the water where circulation is almost non-existent. Light niches are a great example of this. But, because you have copper ions in your water, you are less likely to get an algae bloom and can therefore maintain that lower level of FC....until the day you do get an algae outbreak (it can happen even to the best of us) and then the situation becomes much more complicated.
For those of us that use chlorine alone for sanitation, it would be an impossible task to maintain a pool with no CYA. Yes, one would use less FC, but there would simply be no may to keep the FC level consistent enough in the water volume to be at the proper level for disinfection and the water would need a constant dosing system (like your setup). So, the trade off is to use CYA which allows the pool owner to maintain a higher chlorine residual (so as to never go below the disinfection limit) and to help protect the chlorine from UV photolysis. So in a clean, well managed pool with TFP recommended levels of CYA and FC, TFP users will find that they can drive their FC loss down to 2ppm/day or less (mine is actually less right now and I live in a pretty extreme UV/heat environment) and that makes the water completely manageable for the average pool owner who may only be able to dose their pool once per day with chlorinating liquid.
And finally, if you read that pool water chemistry section carefully, you'll see that
chem geek did qualify the loss rate and half-life charts with the idea that the protective effects of CYA have a component to them which seems to not be dependent on chlorine loss. In other words, based on the anecdotal data we have from users and real world pools, higher CYA levels seem to lower the loss rates more than what would be predicted from the basic chlorine chemistry alone. One theory is that, in deeper waters, CYA has more of a chance to absorb UV directly and re-radiate it at a lower wavelength (either as IR radiation or heat). If that's the case, then most of those charts would show a loss rate higher than what is typically seen when using higher levels of CYA.
Should you add CYA to your pool? Nope. Your pool is different from what we recommend, and because you use an ORP probe, I don't think you should add CYA as that would cause you to have recalibrate it (CYA fouls the ORP probe membranes). I think you should stay-the-course with your pool and see what happens.